PUBLISHER: SNE Research | PRODUCT CODE: 1565778

PUBLISHER: SNE Research | PRODUCT CODE: 1565778
<2024> Technology Development Trends and Prospects of Dry Battery Electrode Process for LIBs
Electric vehicles themselves do not emit greenhouse gases, but the manufacturing process of electric vehicles has been criticized for emitting carbon and destroying the environment. A representative example is the battery, which accounts for about 40% of the manufacturing cost of electric vehicles.
During the battery manufacturing process, a considerable amount of electric energy is consumed, especially in drying and recovering NMP, which is a cause of greenhouse gas emissions. According to one research result, 42 kg of CO2 is generated per kWh due to solvent drying in the wet manufacturing process, and volatile organic compounds (VOCs), which are environmental pollutants, are also emitted into the atmosphere. In contrast, dry electrodes do not have a solvent drying and recovery process, so they consume less electric energy and do not emit VOCs, making them an environmentally friendly process.
In order to increase energy density, a thick film electrode of >100 micrometer or more is required. In the current wet process, it is difficult to make a thick film electrode due to the layer separation phenomenon between the solvent and the material. Since the specific gravity of each material such as the active material, conductive material, and binder is different, if the coating is thick, the binder and conductive material float to the electrode surface. In the wet process, it is difficult to coat the electrode with a thickness of about 100 micrometer or more.
By using a dry process, the active material-conductive material-binder can be evenly distributed without this layer separation phenomenon, so a thick-film electrode can be created, which can increase the capacity and energy density of the battery.
In 2019, Tesla acquired Maxwell Technologies, a supercapacitor company with dry electrode technology, and announced at Battery Day in September 2020 that it would introduce dry electrodes. Tesla sold Maxwell to UCAP in 2021, two years later, but was able to secure dry electrode technology. According to experts who directly obtained and analyzed the Tesla 4680 battery, the battery applied a dry electrode only to the anode, and the existing wet electrode was adopted for the cathode.
It is not known why Tesla has not yet applied the dry electrode process to the cathode, but there is analysis that the yield of the dry electrode process is low and cannot be mass-produced. There are also foreign media reports that the low yield of the 4680 battery is affecting the production of the Cybertruck.
The principle of the dry coating process is simple, but there are considerable challenges at each stage in implementing it in practice. It is not easy to evenly mix the active material, conductive material, and binder without using a solvent. It is even more difficult to evenly apply the non-viscous powder to the current collector. If the yield is low, the production cost increases. Dry electrodes were introduced to reduce costs, but they can actually act as a cost increase factor.
In addition to Tesla, domestic and foreign companies are currently announcing that they are developing a P/P scale dry process, but it is expected that all 46-phi cylindrical batteries to be initially produced will be produced using a wet process. The 4680 battery that LGES will produce in the fourth quarter of 2024 on a P/P scale will apply a wet process to both cathode and anode, and this battery will be supplied to Tesla. Recently, LGES announced that it will complete the construction of a dry electrode process P/P line in the Ochang Energy Plant in the fourth quarter of 2024 and will apply it starting in 2028. Samsung SDI, SK On, Panasonic, CATL, and Kumyang, which recently announced that they are also developing dry electrode technology.
In addition, Volkswagen of Germany announced in June 2023 that it was developing a dry electrode process with Koenig & Bauer, a German printing equipment specialist. Volkswagen plans to start industrial production by 2027. It is not known exactly how Volkswagen and Koenig & Bauer are developing the dry electrode.
The dry process can reduce energy costs by 30% because the drying process is unnecessary, and the area required for drying can be reduced by 50%. The 4680 battery using the dry process can theoretically be cheaper than the LFP battery, but the technology development has not been successful yet.
The introduction of the dry process has great potential as a carbon-neutral process for manufacturing lithium secondary batteries, and the commercialization of dry electrode technology is expected to greatly contribute to reducing battery manufacturing costs while improving performance. Although no company has succeeded in mass production so far, it is very likely that the dry electrode process will become a trend in the near future as major companies are spurring technology development. In addition, the development of the dry electrode process can be applied to the manufacturing process of all-solid-state batteries, which are next-generation batteries. In fact, interest in all-solid-state batteries is increasing both domestically and internationally, and plans for mass production are being established.
This report provides technical information such as the necessity of developing a carbon-neutral process in the secondary battery industry, issues with the existing wet process, and issues with the current dry process, as well as information on recent development trends in dry electrode processes and all-solid-state battery development by many companies, with the aim of forecasting the current and near-future status of the dry process.
Strong Points of This Report:
- 1. Includes rich technical content on the background and development of the dry electrode process
- 2. Includes detailed descriptions of the types of dry electrode processes and electrode process issues
- 3. Includes detailed comparisons of the pros and cons of dry and wet processes as well as battery applications
- 4. Includes detailed technical content on the application of the dry electrode process to the next-generation battery, the all-solid-state battery
- 5. Includes detailed information on the development trends of electrode processes, materials, and equipment companies in the domestic and international industries
- 6. Includes a list of patents related to the dry electrode process of domestic and foreign companies and an analysis of major patents
- 7. Includes research support projects and main contents by country related to dry electrodes
- 8. Includes market outlooks from major research companies on the dry electrode process
[Difference between dry and wet processes for electrode manufacturing]
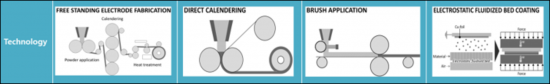
| Advantages | Super caps | compact | ||
| Challenges | Risk of segregation | Risk of segregation | Risk of segregation | mixing |
| Key players | Tesla(Maxwell Technologies | Technical Univ. Dresden Fraunhofer IWS | Technical Univ. Dresden Fraunhofer ISIT | Technical Univ. Braunschweig Fraunhofer IPA |
| TRL | 6 | 5 | 4->6 | 4->6 |
| References | US Patent US2006/0133012A1 10/817 590. Apr. 1. 2008. | Germany Patent DE102017208220A1. Nov. 22. 2018. | Proc. Fraunhofer ISIT - Achievements Results Annu. Rep., 2017.pp.32-33 | Energy Technol., vol. 8, no. 2. 2020. Art. no. 1900309 |
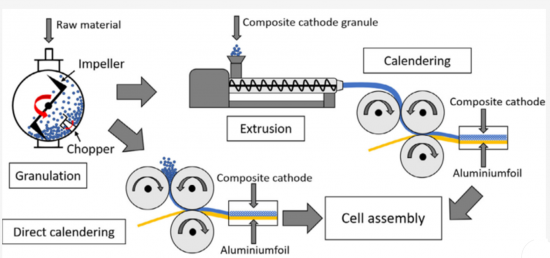
Table of Contents
1. Dry Electrode Processes for Thick Film Electrodes in LIBs
- 1.1. The need for carbon-neutral processes in the LIB industry
- 1.1.1. Increased demand for EV due to carbon neutrality regulations
- 1.1.2. Plans to limit carbon emissions and ban sales of ICE vehicles
- 1.1.3. EV transition plans and verticalized secondary battery companies
- 1.1.4. Industry Issues related to carbon neutrality regulations
- 1.1.5. Secondary battery electrode process, costs and energy consumption
- 1.2. The need for thick film electrodes in Li-ion batteries
- 1.3. Wet-based electrode manufacturing process issues
- 1.4. Background on adopting dry processes
- 1.4.1. Historical and technological advances in dry electrode
- 1.4.2. History of key technology developments in dry film
- 1.4.3. Dry electrode technology: overcoming the limitations of wet coatings
- 1.4.4. Dry process and binder development: number of papers and patents
- 1.4.5. Dry process and binder development: patent analysis
- 1.4.6. Electrode manufacturing with extrusion technology
- 1.4.7. Extrusion and melt processing
- 1.4.8. Melt extrusion: solvent vs. solvent-free application differences
- 1.4.9. Dry electrode application: Tesla anode
- 1.4.10. Dry electrode application: Tesla cathode
- 1.4.11. Dry electrode application: composite cathode
- 1.4.12. Dry electrode application: Pre-lithiation
- 1.4.13. Battery binder characteristics: 7 types compared
- 1.4.14. Types of binders used in dry processes
- 1.4.15. Binder properties used in dry processes
- 1.4.16. Water (PTFE, PAA) vs. oil (PVDF) binders: Performance & tradeoffs
- 1.4.17. Applying PTFE binders
- 1.5. Dry electrode process types
- 1.5.1. Selection of a dry coating process for dry electrodes
- 1.5.2. Comparison and selection of dry coating technologies
- 1.5.3. Dry mixing and coating
- 1.5.4. Comparison of electrochemical behavior of dry and wet electrodes
- 1.5.5. Features of Dry electrode process technologies in LIB application
- 1.5.6. Free standing electrode technology
- 1.5.7. Direct calendaring technology
- 1.5.8. Powder sheeting technology
- 1.5.9. Electrostatic spraying technology
- 1.5.10. Melt deposition technology
- 1.5.11. Powder compaction technology
- 1.5.12. Melt extrusion technology
- 1.6. Issues with dry electrode process
- 1.6.1. Technical hurdles in dry process technology
- 1.6.2. Challenges of dry process in LIB manufacturing
- 1.6.3. Electrical properties of PTFE
- 1.7. Comparison of dry vs. wet processes
- 1.7.1. Comparison of wet and dry process manufacturing technologies
- 1.7.2. Comparison of cell characteristics for dry vs. wet process technologies
- 1.7.3. Disadvantages of the wet process
- 1.7.4. Pros and cons of wet process alternative options
- 1.7.5. Benefits of adopting dry process technology
- 1.7.6. Benefits of adopting dry process technology (speed performance)
- 1.7.7. Benefits of adopting dry process technology (ion channels)
- 1.7.8. Benefits of adopting dry process technology (low cost)
- 1.7.9. Benefits of adopting dry process technology (Machine characteristics)
- 1.7.10. Dry vs. wet characteristics: Applies to cathode, anode
- 1.7.11. Cell performance of electrochemical electrodes
- 1.7.12. Fabrication and characterization of dry electrodes for LIBs
- 1.7.13. Applying dry electrodes : (LFP + CNT + PTFE) cathode
- 1.7.14. Applying dry electrodes : (NCM622 + PVDF) cathode
- 1.7.15. A comprehensive comparison of dry vs. wet process technologies
- 1.8. PTFE fiberization
- 1.8.1. PTFE fiberization reaction
- 1.8.2. PTFE fiberization process
- 1.8.3. PTFE fiberization application
- 1.8.4. Factors affecting PTFE fibrillation
- 1.8.5. Side effects of PTFE binders
- 1.8.6. Blocking the adverse effects of PTFE binders: Graphite surface coat
- 1.8.7. Preparation of graphite anodes by PTFE fiberization method
- 1.8.8. Developing PTFE modified materials
- 1.8.9. Innovative technologies and systems for PTFE-based cells
2. Next Secondary Battery (All-Solid-State Battery) Dry Electrode Processes
- 2.1. Global development trends of solid-state batteries
- 2.1.1. Types and the system configurations of solid-state batteries
- 2.1.2. Design and solutions for high energy density LIBs
- 2.1.3. Dry composite cathode manufacturing methods
- 2.1.4. Overseas all-solid-state battery development trends
- 2.1.5. Korean all-solid-state battery development trends
- 2.2. The need for adopting dry electrode process in solid-state batteries
- 2.3. Examples of dry electrode process applications in solid-state batteries
- 2.3.1. Korean and international companies
- 2.3.2. Korean and international papers
- 2.3.3. Li-S batteries with PTFE
- 2.3.4. Cobalt-free (LNMO) cells with PTFE
- 2.3.5. Solid-state batteries with PTFE (sulfide, oxide, halide)
- 2.3.6. Solid-state electrolyte membranes for solid-state batteries with PTFE
- 2.3.7. Application of inorganic solid electrolytes
- 2.3.8. Application of polymeric solid-state electrolytes
- 2.3.9. Solid-state batteries with dry process (400 Wh/kg)
- 2.3.10. Solid-state batteries with dry process (energy density comparison)
3. Development Trends by Company
- 3.1. Dry electrode process development trends in Korean and international industry
- 3.1.1. International dry process development trends
- 3.1.2. Korean dry process development trends
- 3.1.3. Challenges to dry electrode processes
- 3.1.4. Pros and cons of the dry electrode process
- 3.2. Korean company development trends
- 3.2.1. LG Energy Solutions
- 3.2.2. Samsung SDI
- 3.2.3. SK On
- 3.2.4. Cosmos Lab
- 3.2.5. CNP Solutions
- 3.3. Overseas company development trends
- 3.3.1. TESLA
- 3.3.2. Sakuu (USA)
- 3.3.3. Anaphite (UK)
- 3.3.4. LiCap Technology (USA)
- 3.3.5. AM Batteries (USA)
- 3.3.6. PowerCo SE
- 3.3.7. Dragonfly Energy (USA)
- 3.3.8. ZEON
- 3.3.9. Daikin
- 3.3.10. Chemours (USA)
- 3.3.11. Huacai Technology (China)
- 3.3.12. Baosheng Energy Technology (China)
- 3.3.13. Li Yuanheng (China)
- 3.4. Equipment manufacturer development trends
- 3.4.1. Hanwha Momentum
- 3.4.2. CIS
- 3.4.3. PNT
- 3.4.4. Yunsung F&C
- 3.4.5. NainTech
- 3.4.6. GITech (Korea)
- 3.4.7. KATOP (China)
- 3.4.8. Shanghai Lianjing Automation Technology
- 3.4.9. TOB New battery
- 3.4.10. TMAX Battery Equipment
- 3.4.11. Shenzhen Tsingyan Electronic Technology
- 3.4.12. Huacai Technology
- 3.4.13. ATEIOS System (USA)
- 3.4.14. EIRICH (Germany)
- 3.4.15. Fraunhofer IWS
- 3.5. Development trends in academic and research institutions
- 3.5.1. Korea Institute of Energy Technology
- 3.5.2. Yonsei University
- 3.5.3. Korea University
- 3.5.4. Ulsan Institute of Science and Technology
- 3.5.5. Sungkyunkwan University
- 3.5.6. Gacheon University
- 3.5.7. Fraunhofer ISIT
- 3.5.8. Karlsruhe Institute of Technology (KIT)
- 3.5.9. Dry Coating Forum
4. Patent Analysis
- 4.1. Overseas dry process development patents
- 4.1.1. Overseas dry process development patent list
- 4.1.2. Maxwell Technologies
- 4.1.3. Fraunhofer IWS
- 4.1.4. TESLA
- 4.1.5. Licap New Energy Technologies
- 4.1.6. Dragonfly Energy
- 4.1.7. Anaphite Ltd
- 4.2. Korean dry process development patents
- 4.2.1. LG Chem, LG Energy Solution patents
- 4.2.2. Samsung SDI
- 4.2.3. SK On
- 4.2.4. Hyundai Kia
- 4.2.5. Yunsung F&C
- 4.2.6. Cosmos Lab
- 4.2.7. Korea Ceramic Technology Institute
5. Research Projects by Country
- 5.1. US DOE projects
- 5.1.1. Oak Ridge National Lab
- 5.1.2. NAVITAS Systems
- 5.2. EU projects
- 5.2.1. ELIBAMA program
- 5.2.2. HORIZON Europe : NOVOC project
- 5.2.3. Horizon Europe : BatWoMan
- 5.3. Korean national projects
- 5.3.1. Ministry of Trade and Industry
- 5.3.2. Ministry of Education
- 5.3.3. Ministry of Science and Technology
- 5.3.4. Ministry of Economy and Finance
6. Market outlook (research outlook)
- 6.1. SNE Research
- 6.2. EV Tank
- 6.3. ESP Analysis
- 6.4. Industry ARC
- 6.5. QY Research
- 6.6. Verified Market Reports




