PUBLISHER: Roots Analysis | PRODUCT CODE: 1624834

PUBLISHER: Roots Analysis | PRODUCT CODE: 1624834
Global TCR Therapy Market by Target Indication, Target Antigen, Key Players and Key Geographies : Industry Trends and Global Forecasts, Till 2035
GLOBAL TCR THERAPY MARKET: OVERVIEW
As per Roots Analysis, the global TCR therapy market is estimated to grow from USD 0.03 billion in the current year to USD 4.13 billion by 2035, at a CAGR of 51% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Indications
- Nasopharyngeal Carcinoma
- Multiple Myeloma
- Head and Neck Carcinoma
- Sarcoma
- Melanoma
- Acute Myeloid Leukemia
- Lung Cancer
- Ovarian Cancer
- Merkel Cell Cancer
Target Antigens
- NY-ESO-1
- EBV
- gp100
Key Players
- GlaxoSmithKline
- China Immunotech
- Xinqiao Hospital of Chongqing
- TCRCure Biopharma
- Adaptimmune Therapeutics
- Immunocore
- Intellia Therapeutics
- Takara Bio
- bluebird bio
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and North Africa
- Rest of the World
GLOBAL TCR THERAPY MARKET: GROWTH AND TRENDS
Cancer continues to be one of the key areas of research and drug development within the pharmaceutical industry. In fact, in the past few years, the USFDA has approved more than 70 drugs for the treatment of different types of cancer. Given the challenges associated with conventional therapies, such as non-specificity and several side effects including gastrointestinal and cardiovascular toxicities, drug developers are actively investigating targeted anti-cancer therapies. Of these, modified T-cell receptor (TCR) based therapies have emerged as a promising option. TCR therapy utilizes genetically modified lymphocytes to target specific tumor markers. TCR therapies have been demonstrated to selectively target and eliminate tumor cells from the body of a host with minimal treatment-related side effects. Notably, over 110 clinical trials related to TCR therapies have been registered in the past ten years, indicating substantial research activity. Driven by a promising development pipeline and encouraging clinical trial results, the TCR-based therapy market is likely to witness significant growth in the mid to long-term.
GLOBAL TCR THERAPY MARKET: KEY INSIGHTS
The report delves into the current state of the global TCR therapy market and identifies potential growth opportunities within the industry. Some key findings from the report include:
1. More than 100 industry and non-industry players are currently evaluating the potential of over 190 TCR-based immunotherapies for the treatment of various oncological and non-oncological disorders.
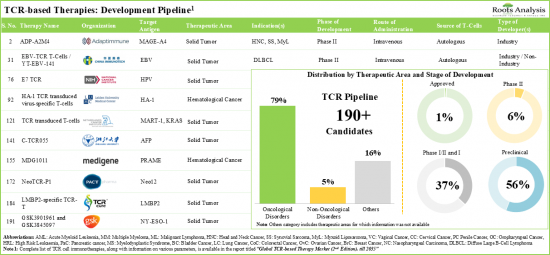
2. More than 90% of the therapy candidates, which are being developed to target a wide range of disease indications are autologous in nature; NY-ESO-1 and MAGE have emerged as the most popular target antigens.
3. In the last 10 years, close to 110 clinical trials have been registered across different geographies for the evaluation of TCR-based therapies; extensive efforts are underway to improve the successive generations of such therapies.
4. Close to 60 scientists from renowned universities are presently involved in the clinical development of TCR-based therapies; majority of these KOLs are primarily based in the US and China.
5. Close to 200 players claim to have the required capabilities to manufacture different types of cell therapies; such firms also offer a wide range of services across different stages of product development.
6. A growing interest in this field is reflected from the increase in the partnership activity, involving both international and indigenous stakeholders; majority of the such deals were signed between players based in North America.
7. Several investors, having realized the opportunity within this upcoming segment of T-cell immunotherapy, have invested USD 11 billion, across 140 instances.
8. More than 75 patents have been filed / granted by various stakeholders in order to protect the intellectual property generated within this field.
9. With a growing focus on the development pipeline and encouraging clinical results, the market is anticipated to witness an annualized growth rate of 51%, in the next decade.
GLOBAL TCR THERAPY MARKET: KEY SEGMENTS
Currently, Multiple Myeloma Segment Occupies the Largest Share of the Global TCR Therapy Market
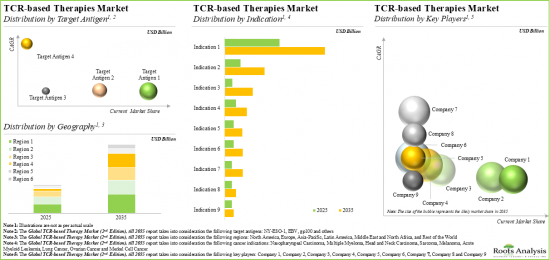
Based on target indication, the market is segmented into nasopharyngeal carcinoma, multiple myeloma, head and neck carcinoma, sarcoma, melanoma, acute myeloid leukemia, lung cancer, ovarian cancer and Merkel cell cancer. At present, the multiple myeloma segment holds the maximum share of the global TCR therapy market. It is worth highlighting that the TCR therapy market for nasopharyngeal carcinoma is likely to drive the market in the near future.
NY-ESO-1 Target Antigen is Likely to Dominate the Global TCR Therapy Market During the Forecast Period
Based on the target antigen, the market is segmented into NY-ESO-1, EBV and gp100. Currently, NY-ESO-1 antigen holds the maximum share within the TCR therapy market. This trend is unlikely to change in the near future.
North America Accounts for the Largest Share of the Market
Based on the key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, Latin America, and the Rest of the world. Majority share is expected to be captured by drug developers based in North America and Europe. It is worth highlighting that, over the years, the market in Asia-Pacific is expected to grow at a higher CAGR.
Example Players in the Global TCR Therapy Market
- Adaptimmune Therapeutics
- Alaunos Therapeutics
- Bristol Myers Squibb
- Cellular Biomedicine
- Gilead Biosciences
- GlaxoSmithKline
- Immatics
- Immunocore
- Lion TCR
- Takara Bio
- Zelluna Immunotherapy
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Vice President, Immuno-Oncology, Celyad
- Vice President, Scientific Affairs, Kite Pharma
- Co-Founder and Chief Executive Officer, Lion TCR
- Chief Operating Officer, TxCell
GLOBAL TCR THERAPY MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global TCR therapy market, focusing on key market segments, including [A] target indications, [B] target antigen, [C] key players and [D] geographical regions.
- Market Landscape: A comprehensive evaluation of TCR therapies, considering various parameters, such as [A] type of developer, [B] phase of development, [C] therapeutic area, [D] key target indication, [E] key target antigen, [F] source of T-cells, [G] route of administration, [H] dosing frequency, [I] target patient segment, [J] type of therapy and [K] most active players (in terms of number of TCR therapies). In addition, the chapter provides details on developer landscape of the industry and non-industry players.
- Popular Target Antigen Analysis: An in-depth analysis of popular target antigens, based on the number of TCR therapies that are being developed for a particular type of antigen by various industry stakeholders to identify potential targets.
- Clinical Trial Analysis: Examination of completed, ongoing, and planned clinical studies of various t-cell receptor therapies based on parameters like [A] trial registration year, [B] enrolled patient population, [C] trial status, [D] trial phase, [E] target patient segment, [F] type of sponsor / collaborator, [G] most active players (in terms of number of registered trials), [H] key focus areas and [I] geography.
- Key Opinion Leader Analysis: An in-depth examination that emphasizes the key opinion leaders (KOLs) within this field includes an evaluation of various principal investigators overseeing clinical trials associated with TCR therapies. In addition, the chapter highlights the most prominent KOLs, based on our proprietary and third-party scoring criteria.
- Drug Profiles: In-depth profiles of marketed and mid- to late-stage clinical products (phase I/II or above), focusing on [A] overview of the therapy, [B] its mechanism of action, [C] dosage information, [D] details on the cost and sales information (wherever available), [E] a clinical development plan, and [F] clinical trial results.
- Company Profiles: In-depth profiles of key industry players in TCR therapies market, focusing on [A] company overviews, [B] product portfolio, [C] technology portfolio (if available), [D] strategic / venture capital investments made in these companies, [E] an insightful recent development related to TCR therapies, and [F] future outlook.
- Partnerships and Collaborations: An analysis of partnerships established in this sector, covering R&D agreements, license agreements (specific to technology platforms and product candidates), product development and commercialization agreements, manufacturing agreements, clinical trial collaborations, product supply management agreements, joint ventures, and others.
- Funding and Investment Analysis: A detailed evaluation of the investments made into the companies having proprietary T-cell receptor candidates / technologies, encompassing seed financing, venture capital financing, capital raised from IPOs and subsequent offerings, grants, and debt financing.
- Patent Analysis: Detailed analysis of various patents filed / granted related to TCR therapies based on [A] type of patent, [B] patent publication year, [C] geographical distribution, [D] Cooperative Patent Classification (CPC) symbols, [E] emerging focus area, [F] type of player, [G] leading player (in terms of number of patents), and [H] patent benchmarking. It also includes a detailed valuation analysis.
- Case Study: A case study on manufacturing cell therapy products, highlighting the key challenges, and a detailed list of contract service providers and in-house manufacturers involved in this space.
- Cost Price Analysis: A comprehensive discussion on the various factors that are likely to influence the pricing of cell-based therapies. This includes an exploration of different models and approaches that pharmaceutical companies may consider while determining the prices of their lead therapy candidates that are likely to be marketed in the near future.
- Promotional Analysis: A review of the key promotional strategies that are being implemented by the developers of the approved TCR therapy (Kimmtrak(R)).
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Chapter Overview
- 1.2. Market Segmentations
- 1.3. Research Methodology
- 1.4. Key Questions Answered
- 1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Pillars of Cancer Therapy
- 3.3. Overview of Immunotherapies
- 3.4. Fundamentals of Cancer Immunotherapy
- 3.5. Classification of Cancer Immunotherapies
- 3.5.1. By Mechanism of Action
- 3.5.1.1. Active Immunotherapy
- 3.5.1.2. Passive Immunotherapy
- 3.5.2. By Type of Target
- 3.5.3. By Approach
- 3.5.3.1. Activation and Suppression Immunotherapy
- 3.5.4. By Product Class
- 3.5.4.1. Monoclonal Antibodies
- 3.5.4.2. Bispecific Antibodies
- 3.5.4.3. Cytokines
- 3.5.4.4. Oncolytic Virus Therapy
- 3.5.4.5. Therapeutic Cancer Vaccines
- 3.5.4.6. Cell-based Therapies
- 3.5.1. By Mechanism of Action
- 3.6. T-Cell Immunotherapies
- 3.6.1. Historical Evolution
- 3.6.2. Key Considerations for Developing T-Cell Immunotherapies
- 3.6.3. Strategies Employed for the Redirection of T-Cells
- 3.6.4. Manufacturing of Engineered T-Cells
- 3.6.5. T-Cell Transduction and Transfection Methods
- 3.6.5.1. Retroviral Vectors
- 3.6.5.2. Lentiviral Vectors
- 3.6.5.3. Non-viral Transfection Methods
- 3.7. T-Cell Receptor (TCR)-based Cell Therapy
- 3.7.1. Development History
- 3.7.2. Anatomical Layout of TCR
- 3.7.3. Development of TCR Therapy
- 3.7.4. Differences between CAR-T and TCR-based Therapies
- 3.8. Concluding Remarks
4. TCR-BASED THERAPIES: MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. TCR-based Therapies: Overall Market Landscape
- 4.2.1. Analysis by Type of Developer
- 4.2.2. Analysis by Phase of Development
- 4.2.3. Analysis by Therapeutic Area
- 4.2.4. Analysis by Phase of Development and Therapeutic Area
- 4.2.5. Analysis by Key Target Indication
- 4.2.6. Analysis by Key Target Antigen
- 4.2.7. Analysis by Source of T-Cells
- 4.2.8. Analysis by Route of Administration
- 4.2.9. Analysis by Phase of Development and Route of Administration
- 4.2.10. Analysis by Dosing Frequency
- 4.2.11. Analysis by Target Patient Segment
- 4.2.12. Analysis by Type of Therapy
- 4.2.13. Analysis by Phase of Development and Type of Therapy
- 4.2.14. Most Active Industry Players: Analysis by Number of TCR-based Therapies
- 4.2.15. Most Active Non-Industry Players: Analysis by Number of TCR-based Therapies
- 4.3. TCR-based Therapies: Overall Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
5. POPULAR TARGET ANTIGEN ANALYSIS
- 5.1. Chapter Overview
- 5.2. TCR-based Therapies: Popular Target Antigens of TCR-based Therapies
- 5.2.1. Popular Targets Related to Hematological Malignancies
- 5.2.2. Popular Targets Related to Solid Tumors
6. CLINICAL TRIAL ANALYSIS
- 6.1. Chapter Overview
- 6.2. Scope and Methodology
- 6.3. TCR-based Therapies: Clinical Trial Analysis
- 6.3.1. Analysis by Trial Registration Year
- 6.3.2. Analysis by Trial Registration Year and Enrolled Patient Population
- 6.3.3. Analysis by Trial Status
- 6.3.4. Analysis by Trial Registration Year and Trial Status
- 6.3.5. Analysis by Trial Phase
- 6.3.6. Analysis of Enrolled Patient Population by Trial Phase
- 6.3.7. Analysis by Target Patient Segment
- 6.3.8. Analysis by Type of Sponsor / Collaborator
- 6.3.9. Analysis by Study Design
- 6.3.10. Most Active Industry Players: Analysis by Number of Registered Trials
- 6.3.11. Most Active Non-Industry Players: Analysis by Number of Registered Trials
- 6.3.12. Word Cloud: Key Focus Areas
- 6.3.13. Analysis of Clinical Trials by Geography
- 6.3.14. Analysis of Enrolled Patient Population by Geography
7. KEY OPINION LEADERS
- 7.1. Chapter Overview
- 7.2. Assumptions and Key Parameters
- 7.3. Methodology
- 7.4. TCR-based Therapies: Key Opinion Leaders
- 7.4.1. Analysis by Type of Organization
- 7.4.2. Analysis by Affiliated Organization
- 7.4.3. Analysis by Qualification
- 7.4.4. Analysis by Geographical Location of KOLs
- 7.4.5. KOL Activeness versus KOL Strength
- 7.4.6. Most Prominent KOLs: Analysis by RA score
- 7.4.7. Most Prominent KOLs: Comparison of RA Score and Third-Party Score
8. TCR-BASED THERAPY PROFILES
- 8.1. Chapter Overview
- 8.2. Kimmtrak(R) / IMCgp100 / Tebentafusp (Immunocore)
- 8.2.1. Therapy Overview
- 8.2.2. Clinical Trial Information
- 8.2.3. Clinical Trial Endpoints
- 8.2.4. Clinical Trial Results
- 8.2.5. Estimated Sales Revenues
- 8.3. GSK3377794 / NY-ESO-1C259 T-cells / Letetresgene Autoleucel (GlaxoSmithKline)
- 8.3.1. Therapy Overview
- 8.3.2. Clinical Trial Information
- 8.3.3. Clinical Trial Endpoints
- 8.3.4. Clinical Trial Results
- 8.3.5. Estimated Sales Revenues
- 8.4. ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics)
- 8.4.1. Therapy Overview
- 8.4.2. Clinical Trial Information
- 8.4.3. Clinical Trial Endpoints
- 8.4.4. Clinical Trial Results
- 8.4.5. Estimated Sales Revenues
- 8.5. JTCR016 (Juno Therapeutics)
- 8.5.1. Therapy Overview
- 8.5.2. Clinical Trial Information
- 8.5.3. Clinical Trial Endpoints
- 8.6. TBI-1301 (Takara Bio)
- 8.6.1. Therapy Overview
- 8.6.2. Clinical Trial Information
- 8.6.3. Clinical Trial Endpoints
- 8.6.4. Clinical Trial Results
- 8.6.5. Estimated Sales Revenues
- 8.7. MDG1011 (Medigene)
- 8.7.1. Therapy Overview
- 8.7.2. Clinical Trial Information
- 8.7.3. Clinical Trial Endpoints
- 8.7.4. Clinical Trial Results
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. TCR-based Therapies: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year of Partnership and Type of Partnership
- 9.3.4. Analysis by Type of Partner
- 9.3.5. Most Popular Products: Analysis by Number of Partnerships
- 9.3.6. Most Active Industry Players: Analysis by Number of Partnerships
- 9.3.7. Most Active Non-Industry Players: Analysis by Number of Partnerships
- 9.3.8. Analysis by Geography
- 9.3.8.1. Intercontinental and Intracontinental Deals
- 9.3.8.2. International and Local Deals
10. FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding
- 10.3. TCR-based Therapies: Funding and Investment Analysis
- 10.3.1. Analysis of Instances by Year
- 10.3.2. Analysis of Amount Invested by Year
- 10.3.3. Analysis by Type of Funding
- 10.3.4. Analysis by Type of Investor
- 10.3.5. Most Active Players: Analysis by Number of Instances
- 10.3.6. Most Active Investors: Analysis by Amount Invested
- 10.3.7. Analysis of Amount Invested by Geography
- 10.3.8. Most Active Investors: Analysis by Number of Funding Instances
11. PATENT ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. TCR-based Therapies: Patent Analysis
- 11.3.1. Analysis by Patent Publication Year
- 11.3.2. Analysis By Patent Application Year
- 11.3.3. Analysis by Geography
- 11.3.4. Analysis by Type of Player
- 11.3.5. Analysis by CPC Symbols
- 11.3.6. Analysis by Key Focus Area
- 11.3.7. Leading Player: Analysis by Number of Patents
- 11.3.8. TCR-based Therapies: Patent Benchmarking
- 11.3.9. Analysis By Patent Characteristics
- 11.3.10. TCR-based Therapies: Patent Valuation
12. CASE STUDY: CELL THERAPY MANUFACTURING
- 12.1. Chapter Overview
- 12.2. Overview of Cell Therapy Manufacturing
- 12.3. Cell Therapy Manufacturing Models
- 12.3.1. Centralized Manufacturing Model
- 12.3.2. Decentralized Manufacturing Model
- 12.4. Scalability of Cell Therapy Manufacturing Processes
- 12.4.1. Scale-Up
- 12.4.2. Scale-Out
- 12.5. Types of Cell Therapy Manufacturers
- 12.6. Key Challenges Related to Manufacturing of Cell Therapies
- 12.7. Important Factors for Cell Therapy Manufacturing
- 12.7.1. Characterization
- 12.7.2. Cost of Goods
- 12.8. Automation of Cell Therapy Manufacturing Processes
- 12.9. Cell Therapy Manufacturing Supply Chain
- 12.10. Comparison of Player Having In-House Capabilities and Contract Manufacturers
- 12.11. Regulatory Landscape
- 12.12. Future Perspectives
13. COST PRICE ANALYSIS
- 13.1. Chapter Overview
- 13.2. Factors Contributing to the High Price of Cell / Gene Therapies
- 13.3. Pricing Models for T-Cell Immunotherapies
- 13.3.1. Based on Associated Costs
- 13.3.2. Based on Availability of Competing Products
- 13.3.3. Based on Patient Segment
- 13.3.4. Based on Opinions of Industry Experts
- 13.4. Reimbursement related Considerations for T-cell Immunotherapies
14. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 14.1. Chapter Overview
- 14.2. Scope and Limitations
- 14.3. Key Assumptions and Forecast Methodology
- 14.4. Global TCR-based Therapies Market, till 2035
- 14.4.1. TCR-based Therapies Market: Distribution by Target Indication
- 14.4.2. TCR-based Therapies Market: Distribution by Target Antigen
- 14.4.3. TCR-based Therapies Market: Distribution by Key Players
- 14.4.4. TCR-based Therapies Market: Distribution by Geography
- 14.4.5. Product Wise Sales Forecast
- 14.4.5.1. Kimmtrak(R) (IMCgp100 / Tebentafusp) (Immunocore)
- 14.4.5.1.1. Sales Forecast (USD Million)
- 14.4.5.1.2. Net Present Value (USD Million)
- 14.4.5.1.3. Value Creation Analysis
- 14.4.5.2. GSK3377794 (GlaxoSmithKline)
- 14.4.5.2.1. Sales Forecast (USD Million)
- 14.4.5.2.2. Net Present Value (USD Million)
- 14.4.5.2.3. Value Creation Analysis
- 14.4.5.3. YT-E001 (China Immunotech)
- 14.4.5.3.1. Sales Forecast (USD Million)
- 14.4.5.3.2. Net Present Value (USD Million)
- 14.4.5.3.3. Value Creation Analysis
- 14.4.5.4. ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics)
- 14.4.5.4.1. Sales Forecast (USD Million)
- 14.4.5.4.2. Net Present Value (USD Million)
- 14.4.5.4.3. Value Creation Analysis
- 14.4.5.5. EBV-specific TCR-T cell with Anti-PD1 Aauto-secreted Element (TCRCure Biopharma)
- 14.4.5.5.1. Sales Forecast (USD Million)
- 14.4.5.5.2. Net Present Value (USD Million)
- 14.4.5.5.3. Value Creation Analysis
- 14.4.5.6. NTLA-5001 (Intellia Therapeutics)
- 14.4.5.6.1. Sales Forecast (USD Million)
- 14.4.5.6.2. Net Present Value (USD Million)
- 14.4.5.6.3. Value Creation Analysis
- 14.4.5.7. TBI-1301 (Takara Bio)
- 14.4.5.7.1. Sales Forecast (USD Million)
- 14.4.5.7.2. Net Present Value (USD Million)
- 14.4.5.7.3. Value Creation Analysis
- 14.4.5.8. LMBP2-specific TCR-T (Xinqiao Hospital of Chongqing / TCR CURE Biopharma Technology)
- 14.4.5.8.1. Sales Forecast (USD Million)
- 14.4.5.8.2. Net Present Value (USD Million)
- 14.4.5.8.3. Value Creation Analysis
- 14.4.5.9. FH-MCVA2TCR (TCRCure Biopharma)
- 14.4.5.9.1. Sales Forecast (USD Million)
- 14.4.5.9.2. Net Present Value (USD Million)
- 14.4.5.9.3. Value Creation Analysis
- 14.4.5.1. Kimmtrak(R) (IMCgp100 / Tebentafusp) (Immunocore)
15. PROMOTIONAL ANALYSIS
- 15.1. Chapter Overview
- 15.2. Channels Used for Promotional Campaigns
- 15.3. Kimmtrak: Promotional Analysis
- 15.3.1. Drug Overview
- 15.3.2. Product Website Analysis
- 15.3.2.1. Message for Healthcare Professionals
- 15.3.2.2. Message for Patients
- 15.3.2.3. Informative Downloads
- 15.3.3. Patient Support Services
16. COMPANY PROFILES
- 16.1. Chapter Overview
- 16.2. Adaptimmune Therapeutics
- 16.3. Alaunos Therapeutics
- 16.4 Bluebird Bio
- 16.5. Bristol Myers Squibb
- 16.6. Cellular Biomedicine Group
- 16.7. Gilead Sciences
- 16.8. GlaxoSmithKline
- 16.9. Immatics
- 16.10. Immunocore
- 16.11. Lion TCR
- 16.12. Takara Bio
- 16.13. Zelluna immunotherapy
17. CONCLUDING REMARKS
18. EXECUTIVE INSIGHTS
- 18.1. Chapter Overview
- 18.2. Celyad
- 18.2.1. Interview Transcript: Vice President, Immuno-Oncology
- 18.3. Kite Pharma
- 18.3.1. Interview Transcript: Vice President, Scientific Affairs
- 18.4. Lion TCR
- 18.4.1. Interview Transcript: Co-Founder and Chief Executive Officer
- 18.5. TxCell
- 18.5.1. Interview Transcript: Chief Operating Officer
19. APPENDIX 1: TABULATED DATA
20. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Types of Immunotherapies and Affiliated Mechanisms of Action
- Table 3.2 FDA Approved Antibody-based Cancer Therapeutics
- Table 3.3 Retroviral Vectors: Salient Features
- Table 3.4 Lentiviral Vectors: Salient Features
- Table 3.5 CAR-T Cell and TCR-based Therapies: Key Differences
- Table 4.1 TCR-based Therapies: Clinical Pipeline
- Table 4.2 TCR-based Therapies: Information on Route of Administration, Source of T-Cells, Dosing Frequency, Target Patient Segment and Type of Therapy
- Table 4.3 TCR-based Therapies: Preclinical Pipeline
- Table 4.4 List of TCR-based Developers
- Table 8.1 TCR-based Cell Therapies: List of Therapies Profiled
- Table 8.2 Therapy Profile: Kimmtrak (Immunocore)
- Table 8.3 Kimmtrak: Clinical Trial Information
- Table 8.4 Kimmtrak: Clinical Trial Endpoints
- Table 8.5 Kimmtrak: Clinical Trial Results
- Table 8.6 Therapy Profile: GSK3377794 (GlaxoSmithKline)
- Table 8.7 GSK3377794: Clinical Trial Information
- Table 8.8 GSK3377794: Clinical Trial Endpoints
- Table 8.9 GSK3377794: Clinical Trial Results
- Table 8.10 Therapy Profile: ADP-A2M4 (Adaptimmune Therapeutics)
- Table 8.11 ADP-A2M4: Clinical Trial Information
- Table 8.12 ADP-A2M4: Clinical Trial Endpoints
- Table 8.13 ADP-A2M4: Clinical Trial Results
- Table 8.14 Therapy Profile: JTCR016 (Juno Therapeutics (Bristol Myers Squibb))
- Table 8.15 JTCR016: Clinical Trial Information
- Table 8.16 JTCR016: Clinical Trial Endpoints
- Table 8.17 Therapy Profile: TBI-1301 (Takara Bio)
- Table 8.18 TBI-1301: Clinical Trial Information
- Table 8.19 TBI-1301: Clinical Trial Endpoints
- Table 8.20 TBI-1301: Clinical Trial Results
- Table 8.21 Therapy Profile: MDG108 (Medigene)
- Table 8.22 MDG108: Clinical Trial Information
- Table 8.23 MDG108: Clinical Trial Endpoints
- Table 8.24 MDG108: Clinical Trial Results
- Table 9.1 List of Partnerships and Collaborations, 2005-2022
- Table 10.1 List of Funding and Investments, 2007-2022
- Table 10.2 Funding and Investment Analysis: Summary of Investments
- Table 11.1 Patent Analysis: Prominent CPC Symbols
- Table 11.2 Patent Analysis: Most Popular CPC Symbols
- Table 11.3 Patent Analysis: List of Top CPC Symbols
- Table 11.4 Patent Analysis: Summary of Benchmarking Analysis
- Table 11.5 Patent Analysis: Categorization based on Weighted Valuation Scores
- Table 12.1 Assessment Strategies for Different Manufacturing Processes
- Table 12.2 Advantages and Disadvantages of Centralized and Decentralized Manufacturing Models
- Table 12.3 Cell Therapy Manufacturing: Companies with In-House Capabilities and Contract Manufacturers
- Table 13.1 Price of Marketed Gene / Cell Therapies
- Table 13.2 Price of Marketed Targeted Drugs
- Table 13.3 TCR-based Therapies: Expert Opinions on Pricing
- Table 13.4 TCR-based Therapies: Reimbursement Landscape
- Table 14.1 TCR-based Therapies: List of Forecasted Molecules
- Table 14.2 Kimmtrak(R) (IMCgp100 / Tebentafusp) (Immunocore): Net Present Value (USD Million)
- Table 14.3 Kimmtrak(R) (IMCgp100 / Tebentafusp) (Immunocore): Value Creation Analysis (USD Million)
- Table 14.4 GSK3377794 (GlaxoSmithKline): Net Present Value (USD Million)
- Table 14.5 GSK3377794 (GlaxoSmithKline): Value Creation Analysis (USD Million)
- Table 14.6 YT-E001 (China Immunotech): Net Present Value (USD Million)
- Table 14.7 YT-E001 (China Immunotech): Value Creation Analysis (USD Million)
- Table 14.8 ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics): Net Present Value (USD Million)
- Table 14.9 ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics): Value Creation Analysis (USD Million)
- Table 14.10 EBV-specific TCR-T cell with anti-PD1 auto-secreted element (TCRCure Biopharma): Net Present Value (USD Million)
- Table 14.11 EBV-specific TCR-T cell with anti-PD1 auto-secreted element (TCRCure Biopharma): Value Creation Analysis (USD Million)
- Table 14.12 NTLA-5001 (Intellia Therapeutics): Net Present Value (USD Million)
- Table 14.13 NTLA-5001 (Intellia Therapeutics): Value Creation Analysis (USD Million)
- Table 14.14 TBI-1301 (Takara Bio): Net Present Value (USD Million)
- Table 14.15 TBI-1301 (Takara Bio): Value Creation Analysis (USD Million)
- Table 14.16 LMBP2-specific TCR-T (Xinqiao Hospital of Chongqing / TCR CURE Biopharma Technology): Net Present Value (USD Million)
- Table 14.17 LMBP2-specific TCR-T (Xinqiao Hospital of Chongqing / TCR CURE Biopharma Technology): Value Creation Analysis (USD Million)
- Table 14.18 FH-MCVA2TCR (TCRCure Biopharma): Net Present Value (USD Million)
- Table 14.19 FH-MCVA2TCR (TCRCure Biopharma): Value Creation Analysis (USD Million)
- Table 15.1 Kimmtrak(R): Drug Overview
- Table 16.1 Leading TCR-based Therapy Developers
- Table 16.2 Adaptimmune Therapeutics: Company Profile
- Table 16.3 Alaunos Therapeutics: Company Profile
- Table 16.4 Bristol Myers Squibb: Company Profile
- Table 16.5 Cellular Biomedicine Group: Company Profile
- Table 16.6 Gilead Sciences: Company Profile
- Table 16.7 GlaxoSmithKline: Company Profile
- Table 16.8 Immatics: Company Profile
- Table 16.9 Immunocore: Company Profile
- Table 16.10 Lion TCR: Company Profile
- Table 16.11 Takara Bio: Company Profile
- Table 16.12 Zelluna Immunotherapy: Company Profile
- Table 19.1 TCR-based Therapies: Distribution by Type of Developer
- Table 19.2 TCR-based Therapies: Distribution by Phase of Development
- Table 19.3 TCR-based Therapies: Distribution by Therapeutic Area
- Table 19.4 TCR-based Therapies: Distribution by Phase of Development and Therapeutic Area
- Table 19.5 TCR-based Therapies: Distribution by Key Target Indications
- Table 19.6 TCR-based Therapies: Distribution by Key Target Antigens
- Table 19.7 TCR-based Therapies: Distribution by Source of T-Cells
- Table 19.8 TCR-based Therapies: Distribution by Phase of Development and Source of T-Cells
- Table 19.9 TCR-based Therapies: Distribution by Route of Administration
- Table 19.10 TCR-based Therapies: Distribution by Dosing Frequency
- Table 19.11 TCR-based Therapies: Distribution by Target Patient Segment
- Table 19.12 TCR-based Therapies: Distribution by Type of Therapy
- Table 19.13 Most Active Industry Players: Analysis by Number of TCR-based Therapies
- Table 19.14 Most Active Non-Industry Players: Distribution by Number of TCR-based Therapies
- Table 19.15 TCR-based Therapy Developers: Distribution by Year of Establishment
- Table 19.16 TCR-based Therapy Developers: Distribution by Company Size
- Table 19.17 TCR-based Therapy Developers: Distribution by Location of Headquarters (Region-wise)
- Table 19.18 TCR-based Therapy Developers: Distribution by Location of Headquarters (Country-wise)
- Table 19.19 Clinical Trial Analysis: Cumulative Distribution of Trials by Trial Registration Year
- Table 19.20 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Registration Year
- Table 19.21 Clinical Trial Analysis: Distribution by Trial Status
- Table 19.22 Clinical Trial Analysis: Distribution by Trial Registration Year and Trial Status
- Table 19.23 Clinical Trial Analysis: Distribution of Trials by Trial Phase
- Table 19.24 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Phase
- Table 19.25 Clinical Trial Analysis: Distribution by Target Patient Segment
- Table 19.26 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 19.27 Clinical Trial Analysis: Distribution by Study Design
- Table 19.28 Most Active Industry Players: Distribution by Number of Registered Trials
- Table 19.29 Most Active Non-Industry Players: Distribution by Number of Registered Trials
- Table 19.30 Clinical Trial Analysis: Distribution of Trials by Geography
- Table 19.31 Clinical Trial Analysis: Distribution of Completed Clinical Trials by Geography
- Table 19.32 Clinical Trial Analysis: Distribution of Active Clinical Trials by Geography
- Table 19.33 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Geography
- Table 19.34 KOL Analysis: Distribution by Type of Organization
- Table 19.35 KOL Analysis: Distribution by Affiliated Organization
- Table 19.36 KOL Analysis: Distribution by Qualification
- Table 19.37 KOL Analysis: Distribution by Geography
- Table 19.38 Most Prominent KOLs: Distribution by RA Score
- Table 19.39 Most Prominent KOLs: Comparison of RA Score with Third-Party Score
- Table 19.40 Kimmtrak(R): Estimated Sales Revenues
- Table 19.41 GSK3377794: Estimated Sales Revenues
- Table 19.42 ADP-A2M4: Estimated Sales Revenues
- Table 19.43 JTCR016: Estimated Sales Revenues
- Table 19.44 TBI-1301: Estimated Sales Revenues
- Table 19.45 MDG1011: Estimated Sales Revenues
- Table 19.46 Partnerships and Collaborations: Cumulative Year-Wise Trend
- Table 19.47 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 19.48 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 19.49 Partnerships and Collaborations: Distribution by Type of Partner
- Table 19.50 Partnerships and Collaborations: Year-Wise Distribution by Type of Partner
- Table 19.51 Most Popular Products: Distribution by Number of Partnerships
- Table 19.52 Most Active Industry Players: Distribution by Number of Partnerships
- Table 19.53 Most Active Non-Industry Players: Distribution by Number of Partnerships
- Table 19.54 Partnerships and Collaborations: Distribution of Intercontinental and Intracontinental Deals
- Table 19.55 Partnerships and Collaborations: Distribution of International and Local Deals
- Table 19.56 Funding and Investment Analysis: Cumulative Distribution of Instances by Year
- Table 19.57 Funding and Investment Analysis: Cumulative Distribution of Amount Invested by Year (USD Million)
- Table 19.58 Funding and Investment Analysis: Distribution of Instances by Type of Funding
- Table 19.59 Funding and Investment Analysis: Distribution of Total Amount Invested by Type of Funding (USD Million)
- Table 19.60 Funding and Investment Analysis: Distribution by Type of Investor
- Table 19.61 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Table 19.62 Most Active Players: Distribution by Number of Instances
- Table 19.63 Most Active Players: Distribution by Amount Invested (USD Million)
- Table 19.64 Funding and Investment Analysis: Distribution of Amount Invested by Geography
- Table 19.65 Patent Analysis: Distribution by Type of Patent
- Table 19.66 Patent Analysis: Cumulative Distribution by Patent Publication Year
- Table 19.67 Patent Analysis: Cumulative Distribution Patent Application Year
- Table 19.68 Patent Analysis: Cumulative Distribution by Annual Granted Patents
- Table 19.69 Patent Analysis: Cumulative Year-wise Distribution of Filed Patent Applications
- Table 19.70 Patent Analysis: Year-wise Distribution of Granted Patents and Patent Applications
- Table 19.71 Patent Analysis: Distribution by Geography
- Table 19.72 Patent Analysis: Distribution by Type of Player
- Table 19.73 Patent Analysis: Distribution by CPC Symbols
- Table 19.74 Leading Industry Players: Distribution by Number of Patents
- Table 19.75 Leading Non-Industry Players: Distribution by Number of Patents
- Table 19.76 Leading Patent Assignees: Distribution by Number of Patents
- Table 19.77 Patent Analysis: Distribution by Patent Age
- Table 19.78 TCR-based Therapies: Patent Valuation Analysis
List of Figures
- Figure 2.1 Executive Summary: Overall Market Landscape
- Figure 2.2 Executive Summary: Clinical Trial Analysis
- Figure 2.3 Executive Summary: Partnerships and Collaborations
- Figure 2.4 Executive Summary: Funding and Investment Analysis
- Figure 2.5 Executive Summary: Patent Analysis
- Figure 2.6 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 3.1 Pillars of Cancer Therapy
- Figure 3.2 Differences between Active and Passive Immunotherapies
- Figure 3.3 Differences between Specific and Non-Specific Immunotherapies
- Figure 3.4 Strategies Employed for the Redirection of T-Cells
- Figure 3.5 T-Cell Manufacturing: General Procedure
- Figure 3.6 TCR-based Therapies: Development Process
- Figure 4.1 TCR-based Therapies: Distribution by Type of Developer
- Figure 4.2 TCR-based Therapies: Distribution by Phase of Development
- Figure 4.3 TCR-based Therapies: Distribution by Therapeutic Area
- Figure 4.4 TCR-based Therapies: Distribution by Phase of Development and Therapeutic Area
- Figure 4.5 TCR-based Therapies: Distribution by Key Target Indications
- Figure 4.6 TCR-based Therapies: Distribution by Key Target Antigens
- Figure 4.7 TCR-based Therapies: Distribution by Source of T-Cells
- Figure 4.8 TCR-based Therapies: Distribution by Phase of Development and Source of T-Cells
- Figure 4.9 TCR-based Therapies: Distribution by Route of Administration
- Figure 4.10 TCR-based Therapies: Distribution by Dosing Frequency
- Figure 4.11 TCR-based Therapies: Distribution by Target Patient Segment
- Figure 4.12 TCR-based Therapies: Distribution by Type of Therapy
- Figure 4.13 Most Active Industry Players: Distribution by Number of TCR-based Therapies
- Figure 4.14 Most Active Non-Industry Players: Distribution by Number of TCR-based Therapies
- Figure 4.15 TCR-based Therapy Developers: Distribution by Year of Establishment
- Figure 4.16 TCR-based Therapy Developers: Distribution by Company Size
- Figure 4.17 TCR-based Therapy Developers: Distribution by Location of Headquarters (Region-wise)
- Figure 4.18 TCR-based Therapy Developers: Distribution by Location of Headquarters (Country-wise)
- Figure 5.1 TCR-based Therapies: Popular Targets in Hematological Malignancies
- Figure 5.2 TCR-based Therapies: Popular Targets in Solid Tumors
- Figure 6.1 Clinical Trial Analysis: Scope and Methodology
- Figure 6.2 Clinical Trial Analysis: Cumulative Distribution of Trials by Trial Registration Year
- Figure 6.3 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Registration Year
- Figure 6.4 Clinical Trial Analysis: Distribution by Trial Status
- Figure 6.5 Clinical Trial Analysis: Distribution by Trial Registration Year and Trial Status
- Figure 6.6 Clinical Trial Analysis: Distribution of Trials by Trial Phase
- Figure 6.7 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Phase
- Figure 6.8 Clinical Trial Analysis: Distribution by Target Patient Segment
- Figure 6.9 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 6.10 Clinical Trial Analysis: Distribution by Study Design
- Figure 6.11 Most Active Industry Players: Distribution by Number of Registered Trials
- Figure 6.12 Most Active Non-Industry Players: Distribution by Number of Registered Trials
- Figure 6.13 Word Cloud Analysis: Emerging Focus Areas
- Figure 6.14 Clinical Trial Analysis: Distribution of Trials by Geography
- Figure 6.15 Clinical Trial Analysis: Distribution of Completed Clinical Trials by Geography
- Figure 6.16 Clinical Trial Analysis: Distribution of Active Clinical Trials by Geography
- Figure 6.17 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Geography
- Figure 7.1 KOL Analysis: Distribution by Type of Organization
- Figure 7.2 KOL Analysis: Distribution by Affiliated Organization
- Figure 7.3 KOL Analysis: Distribution by Qualification
- Figure 7.4 KOL Analysis: Distribution by Geography
- Figure 7.5 TCR-based Therapies Scatter Plot: KOL Activeness versus KOL Strength
- Figure 7.6 Most Prominent KOLs: KOL Activeness versus KOL Strength
- Figure 7.7 Most Prominent KOLs: Distribution by RA Score
- Figure 7.8 Most Prominent KOLs: Comparison of RA Score with Third-Party Score
- Figure 7.9 Most Prominent KOLs: Comparison of RA Score with Third-Party Score (Scatter Plot)
- Figure 8.1 Kimmtrak(R): Estimated Sales Revenues
- Figure 8.2 GSK3377794: Estimated Sales Revenues
- Figure 8.3 ADP-A2M4: Estimated Sales Revenues
- Figure 8.4 JTCR016: Estimated Sales Revenues
- Figure 8.5 TBI-1301: Estimated Sales Revenues
- Figure 8.6 MDG1011: Estimated Sales Revenues
- Figure 9.1 Partnerships and Collaborations: Cumulative Year-Wise Trend
- Figure 9.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.3 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.4 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 9.5 Partnerships and Collaborations: Distribution by Type of Partner
- Figure 9.6 Partnerships and Collaborations: Year-Wise Distribution by Type of Partner
- Figure 9.7 Most Popular Products: Distribution by Number of Partnerships
- Figure 9.8 Most Active Industry Players: Distribution by Number of Partnerships
- Figure 9.9 Most Active Non-Industry Players: Distribution by Number of Partnerships
- Figure 9.10 Partnerships and Collaborations: Intercontinental and Intracontinental Deals
- Figure 9.11 Partnerships and Collaborations: International and Local Deals
- Figure 10.1 Funding and Investment Analysis: Cumulative Year-wise Distribution of Instances
- Figure 10.2 Funding and Investment Analysis: Cumulative Year-wise Distribution of Amount Invested (USD Million)
- Figure 10.3 Funding and Investment Analysis: Distribution of Instances by Type of Funding
- Figure 10.4 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding (USD Million)
- Figure 10.5 Funding and Investment Analysis: Distribution by Type of Investor
- Figure 10.6 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Figure 10.7 Most Active Players: Distribution by Number of Instances
- Figure 10.8 Most Active Players: Distribution by Amount Invested (USD Million)
- Figure 10.9 Funding and Investment Analysis: Distribution of Amount Invested by Geography
- Figure 11.1 Patent Analysis: Distribution by Type of Patent
- Figure 11.2 Patent Analysis: Cumulative Distribution by Patent Publication Year
- Figure 11.3 Patent Analysis: Cumulative Distribution by Patent Application Year
- Figure 11.4 Patent Analysis: Cumulative Year-wise Distribution of Granted Patents
- Figure 11.5 Patent Analysis: Cumulative Year-wise Distribution of Filed Patent Applications
- Figure 11.6 Patent Analysis: Year-wise Distribution of Filed Patent Applications and Granted Patents
- Figure 11.7 Patent Analysis: Distribution by Geography
- Figure 11.8 Patent Analysis: Distribution by Type of Player
- Figure 11.9 Patent Analysis: Distribution by CPC Symbols
- Figure 11.10 Patent Analysis: Key Focus Area
- Figure 11.11 Leading Industry Players: Distribution by Number of Patents
- Figure 11.12 Leading Non-Industry Players: Distribution by Number of Patents
- Figure 11.13 Leading Patent Assignees: Distribution by Number of Patents
- Figure 11.14 Leading Players: Benchmarking by Patent Characteristics (CPC Symbols)
- Figure 11.15 Patent Analysis: Distribution by Patent Age
- Figure 11.16 TCR-based Cell Therapies: Patent Valuation Analysis
- Figure 12.1 Steps for Manufacturing Cell Therapies
- Figure 12.2 Centralized Manufacturing: Process Model
- Figure 12.3 Decentralized Manufacturing: Process Model
- Figure 12.4 Cell Therapy Manufacturing: Types of Manufacturers
- Figure 12.5 Cell Therapy: Challenges and Drivers
- Figure 12.6 Cell Therapies: Potency as Critical Quality Attribute
- Figure 12.7 Cell Therapy Manufacturing: Supply Chain Model
- Figure 12.8 Cell Therapy Manufacturing: Supply Chain Risk Assessment Considerations
- Figure 13.1 Approved T-Cell Therapies: Pricing Model based on Patient Segment
- Figure 14.1 Global TCR-based Therapies Market, till 2035 (USD Billion)
- Figure 14.2 TCR-based Therapies Market: Distribution by Target Indication, Current Year and 2035 (USD Billion)
- Figure 14.3 TCR-based Therapies Market: Distribution by Target Antigen, Current Year and 2035 (USD Billion)
- Figure 14.4 TCR-based Therapies Market: Distribution by Key Players, Current Year and 2035 (USD Billion)
- Figure 14.5 TCR-based Therapies Market: Distribution by Geography, Current Year and 2035 (USD Billion)
- Figure 14.6 Kimmtrak(R) (IMCgp100 / Tebentafusp) (Immunocore) Sales Forecast, till 2035 (USD Million)
- Figure 14.7 GSK3377794 (GlaxoSmithKline) Sales Forecast, till 2035 (USD Million)
- Figure 14.8 YT-E001 (China Immunotech) Sales Forecast, till 2035 (USD Million)
- Figure 14.9 ADP-A2M4 / Afamitresgene Autoleucel / Afami-cel (Adaptimmune Therapeutics) Sales Forecast, till 2035 (USD Million)
- Figure 14.10 EBV-specific TCR-T cell with anti-PD1 auto-secreted element (TCRCure Biopharma) Sales Forecast, till 2035 (USD Million)
- Figure 14.11 NTLA-5001 (Intellia Therapeutics) Sales Forecast, till 2035 (USD Million)
- Figure 14.12 TBI-1301 (Takara Bio) Sales Forecast, till 2035 (USD Million)
- Figure 14.13 LMBP2-specific TCR-T (Xinqiao Hospital of Chongqing / TCR CURE Biopharma Technology) Sales Forecast, till 2035 (USD Million)
- Figure 15.1 Channels Used for Promotional Campaigns
- Figure 15.2 Product Website Analysis: Kimmtrak(R), Messages for Healthcare Professional
- Figure 15.3 Product Website Analysis: Kimmtrak(R), Messages for Patients
- Figure 15.4 Product Website Analysis: Kimmtrak(R), Kimmtrak Connect
- Figure 17.1 Concluding Remarks: Market Landscape
- Figure 17.2 Concluding Remarks: Clinical Trial Analysis
- Figure 17.3 Concluding Remarks: Key Opinion Leaders
- Figure 17.4 Concluding Remarks: Partnerships & Collaborations
- Figure 17.5 Concluding Remarks: Funding and Investment Analysis
- Figure 17.6 Concluding Remarks: Patent Analysis
- Figure 17.7 Concluding Remarks: Market Forecast




