PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1708209

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1708209
Clinical Trial Packaging Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
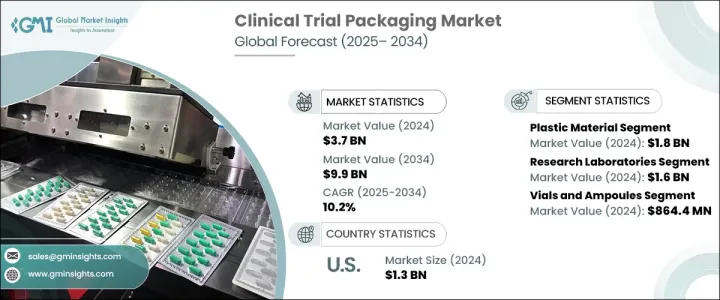
The Global Clinical Trial Packaging Market generated USD 3.7 billion in 2024 and is anticipated to grow at a CAGR of 10.2% from 2025 to 2034, driven by increasing demand for biologics, complex drug formulations, and a growing number of clinical trials conducted worldwide. As the pharmaceutical industry evolves, there is a clear trend toward smaller, more customized batches, creating a strong need for flexible and scalable packaging solutions. The rising focus on personalized medicine and targeted therapies, including gene therapies and mRNA vaccines, is accelerating the demand for specialized packaging that ensures product stability and safety during transportation and storage.
Additionally, the emergence of decentralized clinical trials (DCTs) is adding complexity to packaging requirements, with manufacturers prioritizing versatile, easy-to-manage solutions that cater to diverse trial formats. Stringent regulatory standards further emphasize the need for packaging that maintains the integrity of temperature-sensitive biologics while ensuring compliance with international safety guidelines. Advancements in packaging technologies, including the use of smart packaging systems equipped with real-time monitoring features, are enhancing the efficiency and reliability of clinical trial supply chains.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.7 Billion |
| Forecast Value | $9.9 Billion |
| CAGR | 10.2% |
The market is segmented by material type, including plastic, glass, metal, and paper-based packaging. Among these, the plastic segment generated USD 1.8 billion in 2024, owing to its lightweight, cost-effective nature, making it ideal for large-scale shipping and storage. Plastic packaging is highly adaptable, allowing manufacturers to customize clinical trial kits and small batch formats easily. This versatility is especially beneficial for accommodating prefilled syringes, pouches, and other pharmaceutical products that require flexibility and ease of production. As the pharmaceutical landscape increasingly demands precision and variability in packaging, plastic remains the preferred choice for supporting a wide range of clinical trial packaging requirements.
Research laboratories emerged as the largest end-user segment in the clinical trial packaging market, generating USD 1.6 billion in 2024. These laboratories play a pivotal role in the clinical trial ecosystem, particularly during the early stages of drug development, where specialized packaging is required for small quantities of various drug formulations. The growing emphasis on biologics and personalized therapies, including gene therapies and targeted treatments, has further fueled the demand for sterile, temperature-sensitive packaging solutions. Glass vials and cryogenic containers are particularly essential for preserving the stability of sensitive formulations, ensuring the safety and efficacy of drugs during clinical trials.
North America held a dominant 41.5% share of the clinical trial packaging market in 2024, driven by the increasing number of clinical trials focused on personalized medicine, biologics, and treatments for rare diseases. Government investments in decentralized clinical trials and cold chain infrastructure are accelerating the demand for specialized packaging solutions across the region. The rising prevalence of chronic diseases, coupled with ongoing advancements in biologics and cell-based therapies, is prompting pharmaceutical companies to invest heavily in innovative packaging solutions to meet evolving clinical trial requirements. These developments are expected to fuel further growth and innovation in the clinical trial packaging industry, particularly in North America, where a robust healthcare infrastructure and regulatory support create a conducive environment for market expansion.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing R&D investment by pharmaceutical companies
- 3.2.1.2 Stringent packaging regulations for drug safety
- 3.2.1.3 Growth in biologics and complex drug formulations
- 3.2.1.4 Growing number of clinical trials
- 3.2.1.5 Rising prevalence of chronic diseases
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of specialized packaging
- 3.2.2.2 Logistical constraints in cold chain management
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Material Type, 2021 - 2034 (USD Million & Kilo Tons)
- 5.1 Key trends
- 5.2 Plastic
- 5.3 Glass
- 5.4 Metal
- 5.5 Paper & paperboard
Chapter 6 Market Estimates and Forecast, By Product Type, 2021 - 2034 (USD Million & Kilo Tons)
- 6.1 Key trends
- 6.2 Syringes
- 6.3 Vials & ampoules
- 6.4 Blisters
- 6.5 Tubes
- 6.6 Bottles
- 6.7 Bags & pouches
- 6.8 Kits and packs
- 6.9 Others
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 (USD Million & Kilo Tons)
- 7.1 Key trends
- 7.2 Research laboratories
- 7.3 Clinical research organizations
- 7.4 Drug manufacturing facilities
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 (USD Million & Kilo Tons)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 Saudi Arabia
- 8.6.2 South Africa
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Adare Pharma Solutions
- 9.2 Adelphi Healthcare Packaging
- 9.3 Almac Group
- 9.4 Amcor
- 9.5 AptarGroup
- 9.6 Berry Global
- 9.7 Brentwood Industries
- 9.8 Caprihans India
- 9.9 CCL Industries
- 9.10 DWK Life Sciences
- 9.11 Gerresheimer
- 9.12 Korber
- 9.13 Parexel International
- 9.14 Schott
- 9.15 SGD Pharma
- 9.16 Sharp Services
- 9.17 TA Instruments
- 9.18 WestRock
- 9.19 Yourway




