PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1665063

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1665063
Oncology Based In-vivo CRO Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
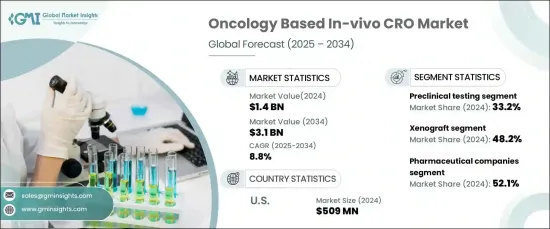
The Global Oncology Based In-Vivo CRO Market reached USD 1.4 billion in 2024 and is expected to grow at a robust compound annual growth rate (CAGR) of 8.8% from 2025 to 2034. This significant market expansion is being driven by the increasing demand for immuno-oncology therapies, ongoing advancements in in-vivo models, and a rise in oncology drug approvals from both biotechnology and pharmaceutical companies.
Small biotechnology and pharmaceutical companies are playing a crucial role in driving the development of oncology drugs, thus accelerating overall market growth. These companies focus on innovative treatments, including gene therapies and antibody-based drugs, which are designed to address unmet medical needs in the oncology space. Their specialized approach to cancer research continues to fuel the demand for preclinical and in-vivo testing services, further contributing to the market's upward trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $3.1 Billion |
| CAGR | 8.8% |
The oncology in-vivo CRO market is segmented by service type, with the primary categories being preclinical testing, efficacy testing, toxicology studies, pharmacokinetics, and others. Preclinical testing held the largest share of the market in 2024, accounting for 33.2%. This dominance is due to the vital role preclinical testing plays in drug development, ensuring the safety and efficacy of new therapies before they progress to human clinical trials. Regulatory authorities such as the U.S. FDA and EMA require preclinical testing as a critical step before clinical trials can commence. These studies provide essential data on drug toxicity, pharmacodynamics, and therapeutic effects, making them indispensable in the oncology research pipeline.
The market is also segmented by model type, including xenograft, syngeneic, and other models. Xenograft models led the market in 2024, holding a 48.2% share. These models are widely preferred due to their ability to closely mimic human tumor biology, offering crucial insights into the effectiveness of cancer drugs. Xenograft models involve implanting human tumor tissues into immunocompromised mice, which enables an accurate simulation of human cancer progression and enhances the evaluation of drug efficacy.
In the United States, the oncology in-vivo CRO market accounted for USD 509 million in 2024, dominating the regional landscape. The country's market leadership can be attributed to significant investments in oncology research and the strength of its pharmaceutical and biotechnology sectors. With numerous companies focused on oncology drug development, the U.S. heavily relies on outsourcing preclinical and in-vivo studies to CROs. This growing trend supports continued market expansion, driven by innovations in oncology therapies and a rising demand for in-vivo testing solutions.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope & definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates & calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing demand for immuno-oncology therapies
- 3.2.1.2 Advancements in in-vivo models
- 3.2.1.3 Growing investments in precision oncology
- 3.2.1.4 Growing oncology drug approvals from small biotechnology and pharmaceutical companies
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 Stringent regulatory requirements for oncology drug approvals
- 3.2.2.2 Presence of strong alternatives
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Patent Analysis
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Service, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Preclinical testing
- 5.3 Efficacy testing
- 5.4 Toxicology studies
- 5.5 Pharmacokinetics
- 5.6 Other services
Chapter 6 Market Estimates and Forecast, By Model, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Xenograft
- 6.2.1 Patient derived xenograft
- 6.2.2 Cell-line derived xenograft
- 6.2.3 Other xenografts
- 6.3 Syngeneic
- 6.4 Other models
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical companies
- 7.3 Biotechnology companies
- 7.4 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Charles River Laboratories
- 9.2 Crown Bioscience
- 9.3 Eurofins Scientific
- 9.4 Evotec SE
- 9.5 ICON plc
- 9.6 IMV
- 9.7 Laboratory Corporation of America Holdings
- 9.8 Medidata
- 9.9 Merck KGaA
- 9.10 OncoOne
- 9.11 Pharmaron
- 9.12 Taconic Biosciences
- 9.13 The Jackson Laboratory
- 9.14 Thermo Fisher Scientific
- 9.15 WuXi AppTec




