PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1674080

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1674080
U.S. Transthyretin Amyloidosis Treatment Market, By Type, By Drug Type, By Distribution Channel
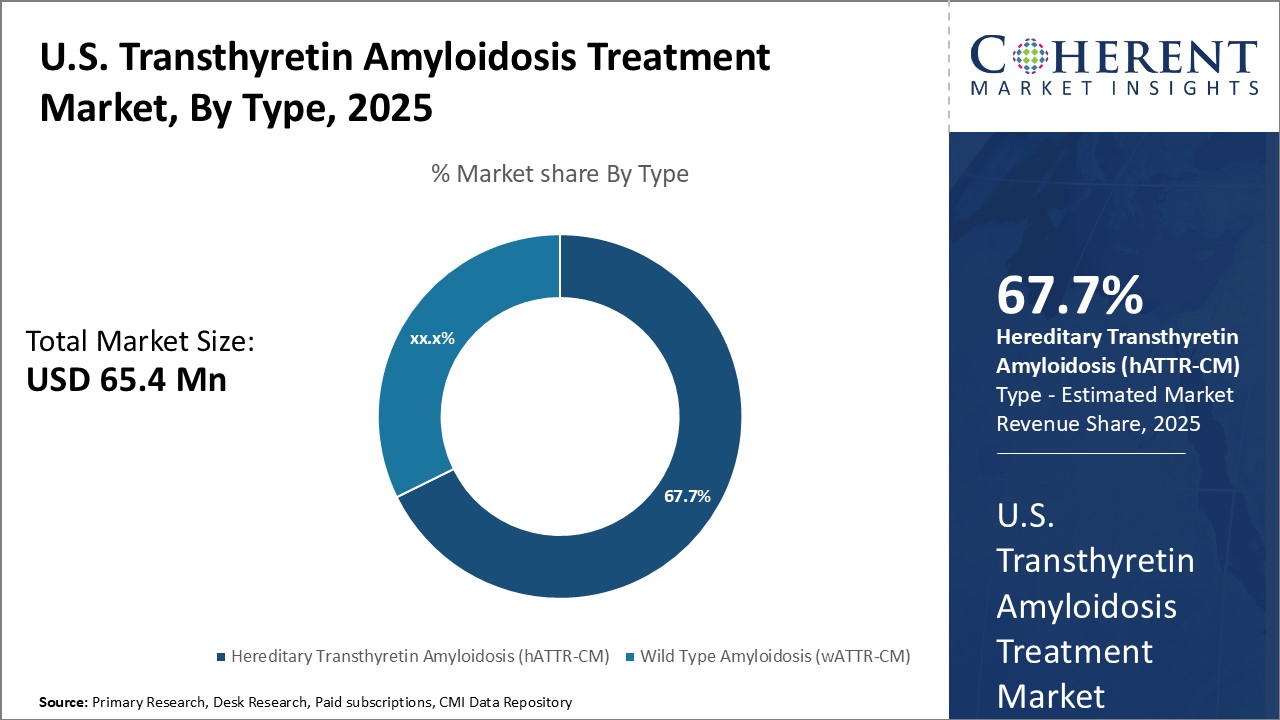
Global U.S. Transthyretin Amyloidosis Treatment Market is estimated to be valued at USD 65.4 Mn in 2025 and is expected to reach USD 124.4 Mn by 2032, growing at a compound annual growth rate (CAGR) of 9.6% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 65.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.60% | 2032 Value Projection: | USD 124.4 Mn |
Transthyretin amyloidosis is a rare, progressive, and fatal disorder caused by mutations in the transthyretin (TTR) gene. These mutations lead to deposition of abnormal TTR protein fibers known as amyloid in vital organs like heart, nerves, and gastrointestinal tract. The accumulation of amyloid fibrils in these organs progressively damages their structure and function. With no approved disease-modifying therapies available so far, ATTR amyloidosis was considered untreatable and fatal. However, recent advancements in understanding the disease pathogenesis along with approvals of novel therapies have provided new hope to patients. The U.S. transthyretin amyloidosis treatment market has witnessed significant growth due to these approval milestones.
Market Dynamics:
The U.S. transthyretin amyloidosis treatment market growth is driven by increasing disease awareness, approvals of novel disease-modifying therapies, and expansion of treatment landscape beyond symptom management. Factors such as longer life expectancy and improved diagnosis are also contributing to market growth. Despite the positive momentum, high treatment costs remain a key restraint. Infrastructure challenges in effective diagnosis also hamper market potential. However, ongoing clinical trials evaluating new treatment options and expansion of approval to different subtypes present lucrative opportunities. Partnerships between players to enhance access further boost the market outlook.
Key Features of the Study:
This report provides in-depth analysis of the U.S. transthyretin amyloidosis treatment market, and provides market size (US$ Mn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
Key companies covered as a part of this study include Alnylam Pharmaceuticals, Inc., Pfizer, Inc., Prothena Corporation Plc, GSK plc, Ionis Pharmaceuticals, Inc., SOM Innovation Biotech, S.L., Eidos Therapeutics, BridgeBio Pharma, Inc., Bristol-Myers Squibb Company, AstraZeneca, Acrotech Biopharma, and Attralus, Inc.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The U.S. transthyretin amyloidosis treatment market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the U.S. transthyretin amyloidosis treatment market
Market Segmentation
- By Type Insights (Revenue, USD Mn, 2019 - 2032)
- Hereditary Transthyretin Amyloidosis (hATTR-CM)
- Wild Type Amyloidosis (wATTR-CM)
- By Drug Type Insights (Revenue, USD Mn, 2019 - 2032)
- Tafamidis
- Diflunisal
- Inotersen
- Patisiran
- Tolcapone
- Others
- By Distribution Channel Insights (Revenue, USD Mn, 2019 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Key Players Insights
- Alnylam Pharmaceuticals, Inc.
- Pfizer, Inc.
- Prothena Corporation Plc
- GSK plc
- Ionis Pharmaceuticals, Inc.
- SOM Innovation Biotech, S.L.
- Eidos Therapeutics
- BridgeBio Pharma, Inc.
- Bristol-Myers Squibb Company
- AstraZeneca
- Acrotech Biopharma
- Attralus, Inc.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- U.S. Transthyretin Amyloidosis Treatment Market, By Type
- U.S. Transthyretin Amyloidosis Treatment Market, By Drug Type
- U.S. Transthyretin Amyloidosis Treatment Market, By Distribution Channel
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Epidemiology
4. U.S. Transthyretin Amyloidosis Treatment Market, By Type, 2020-2032, (USD Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hereditary Transthyretin Amyloidosis (hATTR-CM)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Wild Type Amyloidosis (wATTR-CM)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
5. U.S. Transthyretin Amyloidosis Treatment Market, By Drug Type, 2020-2032, (USD Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Tafamidis
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Diflunisal
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Inotersen
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Patisiran
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Tolcapone
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
6. U.S. Transthyretin Amyloidosis Treatment Market, By Distribution Channel, 2020-2032, (USD Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Mn)
7. Competitive Landscape
- Alnylam Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Pfizer, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Prothena Corporation Plc
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- GSK plc
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Ionis Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- SOM Innovation Biotech, S.L.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Eidos Therapeutics
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- BridgeBio Pharma, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Bristol-Myers Squibb Company
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- AstraZeneca
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Acrotech Biopharma
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Attralus, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
8. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
9. References and Research Methodology
- References
- Research Methodology
- About us




