PUBLISHER: Roots Analysis | PRODUCT CODE: 1624836

PUBLISHER: Roots Analysis | PRODUCT CODE: 1624836
Prefilled Syringes Market by Purpose of Syringe, Therapeutic Area, Type of Molecule, Type of Barrel Material, Usability of Syringe, Type of Syringe, Type of Packaging and Key Geographical Regions: Industry Trends and Global Forecasts, Till 2035
PREFILLED SYRINGES MARKET: OVERVIEW
As per Roots Analysis, the global prefilled syringes market is estimated to grow from USD 3.02 billion in the current year to USD 4.97 billion by 2035, at a CAGR of 4.6% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Purpose of Syringe
- Specialty Prefilled Syringes
- Therapeutic Prefilled Syringes
- Cosmetic Prefilled Syringes
Therapeutic Area (Therapeutic Syringes)
- Blood Disorders
- Infectious Diseases
- Autoimmune Disorders
- Oncological Disorders
- Cardiovascular Disorders
- Respiratory Disorders
- Neurological Disorders
- Metabolic Disorders
- Ophthalmic Disorders
- Orthopedic Disorders
- Others
Type of Molecule (Therapeutic Syringes)
- Proteins
- Antibodies
- Peptides
- Small Molecules
- Vaccines
- Cell Therapies
Type of Barrel Material
- Glass Syringe
- Plastic Syringe
Number of Barrel Chamber
- Single Chamber Syringe
- Dual Chamber Syringe
Type of Needle System
- Staked Needle Syringe
- Luer Syringe
Usability of Syringe
- Disposable Syringe
- Reusable Syringe
Type of Syringe
- Conventional Syringe
- Safety Syringe
Type of Packaging
- Bulk Syringe
- Nested Syringe
Key Geographical Regions
- North America (US and Canada)
- Europe (Germany, France, Italy, Spain, UK, Rest of Europe)
- Asia-Pacific (Japan, India, China, Australia, South Korea, Rest of Asia Pacific)
- Middle East and North Africa
- Latin America
PREFILLED SYRINGES MARKET: GROWTH AND TRENDS
With the increasing prevalence of chronic diseases and the growing need to administer injectable medications on a frequent basis, there is a notable rise in preference for self-administration of drugs / therapeutics. According to a recent study, close to 133 million individuals annually suffer from one or more chronic disorders in the US alone and this number is likely to reach 170 million by 2030. As a result, a number of drug delivery devices that can be used for self-medication have been developed and introduced into the market. Examples include autoinjectors, large volume wearable injectors, pen injectors and prefilled syringes. It is worth highlighting that among the aforementioned devices, prefilled syringes are the most established range of products. In fact, given the advantages it offers over other traditional drug administration solutions, the applications of this drug packaging and delivery system have increased over the years. Moreover, with the increase in development of parenteral drugs, the consumption of prefilled syringes has tripled. The current demand for safer, easier to administer technologies along with the industry's efforts to reduce costs and increase efficiency are expected to drive the growth of the prefilled syringes market in the future.
PREFILLED SYRINGES MARKET: KEY INSIGHTS
The report delves into the current state of the prefilled syringes market and identifies potential growth opportunities within the industry. Some key findings from the report include:
1. Presently, more than 120 prefilled syringes are available / being manufactured across the world; majority of the prefilled syringe manufacturers are based in Asia-Pacific.
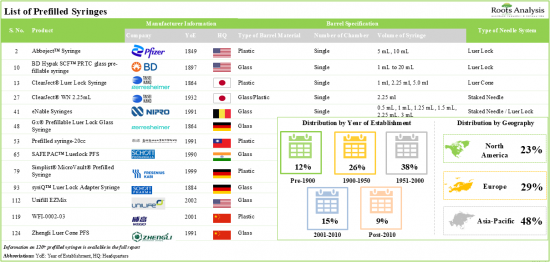
2. Prefilled syringes are developed using different materials, needle systems and in different volume formats, to cater to the preferences and requirements of clients in the healthcare industry.
3. In pursuit of obtaining a competitive edge, companies are actively undertaking initiatives to integrate advanced features in their respective prefilled syringe portfolios.
4. Since 2019, more than 1,300 patents related to prefilled syringes and its related components have been filed / granted by various stakeholders to protect the intellectual property generated within this domain.
5. Several scientists, clinicians and industry veterans, affiliated to academic / medical / commercial organizations, are spearheading research related to prefilled syringes.
6. More than 170 prefilled syringe combination products are either commercially available or being evaluated for a variety of dosage forms.
7. Around 70% of the prefilled syringe combination products are currently in the later phases of development (Phase III and IV); the majority of these are being administered through the subcutaneous route.
8. Several big pharma players are actively carrying out initiatives to strengthen their injectable drug pipeline, across various therapeutic areas.
9. Close to 50 players are engaged in offering fill / finish services for prefilled syringes; majority of these players are based in Europe, followed by North America.
10. Identifying the driving factors (that fuel advancements) as well as barriers (that slow down the research progress) helps improve strategic planning and results in efficient operations in the prefilled syringes domain.
11. Owing to the growing demand for biologics and biosimilars (requiring precise dosing), and higher adoption of self-injection devices (in emergency conditions), the prefilled syringes market is likely to grow at a steady rate, till 2035.
12. Glass prefilled syringes capture majority share of this market; dual chamber prefilled syringes are anticipated to grow at a faster pace as compared to single chamber prefilled syringes.
13. The projected future opportunity for prefilled syringes market is likely to be well distributed in terms of usability of syringe, type of syringe, type of packaging and key geographical regions.
PREFILLED SYRINGES MARKET: KEY SEGMENTS
Currently, Specialty Syringes Segment Occupies the Largest Share of the Prefilled Syringes Market
Based on the purpose of the syringe, the market is segmented into specialty syringes, therapeutic syringes and cosmetic pre-filled syringes. At present, the specialty syringes segment holds the maximum share of the prefilled syringes market. It is worth highlighting that the prefilled syringes market for cosmetic pre-filled syringes is likely to grow at a relatively higher CAGR, during the forecast period.
Blood Disorders Segment Accounts for the Largest Share of the Prefilled Syringes Market
Based on the therapeutic area (therapeutic syringes), the market is segmented into blood disorders, infectious diseases, autoimmune disorders, oncological disorders, cardiovascular disorders, respiratory disorders, neurological disorders, metabolic disorders, ophthalmic disorders, orthopedic disorders and others. While blood disorders account for a relatively higher market share, it is worth highlighting that the oncological disorders segment is expected to witness substantial market growth in the coming years.
Protein Molecules are Likely to Dominate the Prefilled Syringes Market During the Forecast Period
Based on the type of molecule, the market is segmented into proteins, antibodies, peptides, small molecules, vaccines and cell therapies. At present, protein molecules hold the maximum share of the prefilled syringes market. It is worth highlighting that the prefilled syringes market for cell therapy is likely to grow at a relatively higher CAGR, during the forecast period.
Glass Material Accounts for the Largest Share of the Prefilled Syringes Market
Based on the type of barrel material, the market is segmented into glass syringes and plastic syringes. Currently, glass syringes hold the maximum share of the prefilled syringes market. It is worth highlighting that the prefilled syringes market for plastic syringes is likely to grow at a relatively higher CAGR, during the forecast period.
Currently, Single Chambered Segment Occupies the Largest Share of the Prefilled Syringes Market
Based on the number of barrel chambers, the market is segmented into single chambered and dual chambered. At present, glass syringes hold the maximum share of the prefilled syringes market. It is worth highlighting that the prefilled syringes market for dual chambered is likely to grow at a relatively higher CAGR, during the forecast period.
Luer Syringes Segment is the Fastest Growing Segment of the Prefilled Syringes Market During the Forecast Period
Based on the type of needle system, the market is segmented into staked needle syringes and luer syringes. At present, the staked needle syringe segment holds the maximum share of the prefilled syringes market. This can be attributed to the fact that these syringes have a low risk of needle detachment and are preferred in emergency medical conditions. It is worth highlighting that the prefilled syringes market for luer syringes is likely to grow at a relatively higher CAGR, during the forecast period.
Currently, Disposable Syringes Segment Occupies the Largest Share of the Prefilled Syringes Market
Based on the usability of syringes, the market is segmented into disposable syringes and reusable syringes. At present, disposable syringes hold maximum share of the prefilled syringes market. It is worth highlighting that the prefilled syringes market for reusable syringes is likely to grow at a relatively higher CAGR, during the forecast period.
Safety Prefilled Syringes Segment is Likely to Dominate the Prefilled Syringes Market During the Forecast Period
Based on the types of syringes, the market is segmented into conventional syringes and safety syringes. It is worth highlighting that, at present, safety prefilled syringes hold a larger share in the prefilled syringes market. This trend is likely to remain the same in the coming decade.
Currently, Nested Syringe Segment Occupies the Largest Share of the Prefilled Syringes Market
Based on the type of packaging, the market is segmented into nested syringe and bulk syringe. It is worth highlighting that, at present, nested syringe holds larger share in the prefilled syringes market. This trend is likely to remain the same in the coming decade.
Europe Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. It is worth highlighting that, over the years, the market in Asia-Pacific is expected to grow at a higher CAGR.
Example Players in the Prefilled Syringes Market
- Becton Dickinson
- Credence MedSystems
- Gerresheimer
- J.O.Pharma
- Medefil
- MedXL
- Mitsubishi Gas Chemical
- Nipro PharmaPackaging
- Novartis
- Pfizer
- SCHOTT
- Shandong Pharmaceutical Glass
- Shandong Weigao
- Shin Yan Sheno Precision Industrial
- Stevanato
- Taisei Kako
- Vetter Pharma
- West Pharmaceutical
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Founder and Chief Technology Officer, Oval Medical Technologies
- Chief Technical Officer, Intas Pharmaceuticals
- Senior Director and Former Director, West Pharmaceutical
- Head of R&D and Product Strategy, Lonstroff
- Senior Portfolio Director, IDEO
- Former Chief Commercial Officer, IDT Biologika
- Chief Executive Officer, A Small Medical Device Company
PREFILLED SYRINGES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the prefilled syringes market, focusing on key market segments, including [A] purpose of syringe, [B] therapeutic area (therapeutic syringes), [C] type of molecule (therapeutic syringes), [D] type of barrel material, [E] number of barrel chamber, [F] type of needle system, [G] usability of syringe, [H] type of syringe, [I] type of packaging and [J] key geographical regions.
- Market Landscape: A comprehensive evaluation of the current prefilled syringes market, considering various parameters, such as [A] type of barrel fabrication material, [B] number of barrel chamber, [C] type of needle system and [D] volume of syringe. Further, the chapter provides a detailed analysis of prefilled syringe manufacturers, along with information on their [E] year of establishment, [F] company size (in terms of employee count), [G] location of headquarters, [H] location of their manufacturing facilities and [I] most active players (in terms of number of prefilled syringes manufactured).
- Product Competitiveness Analysis: A comprehensive competitive analysis of prefilled syringes, examining factors, such as [A] manufacturer strength and [B] product strength and [C] number of type of needle system.
- Company Profiles: In-depth profiles of key prefilled syringes manufacturers based in North America, Europe and Asia Pacific, focusing on [A] company overviews, [B] financial information (if available) [C] prefilled syringes portfolio, [D] recent developments and [D] an informed future outlook.
- Regulatory Landscape: A discussion on general regulatory guidelines for the approval of prefilled syringes, across different countries / geographical regions.
- Combination Products Analysis: A comprehensive examination of various combination products currently under evaluation or available as prefilled syringes, with a primary emphasis on injectable medications and vaccines. This analysis includes a thorough review of approved drugs, based on several relevant parameters, including [A] type of drug molecule, [B] year of approval, [C] region of approval, [D] route of administration and [E] target therapeutic area. Additionally, it includes analysis for products that are in clinical stage, for parameters such as [F] type of drug molecule, [G] phase of development, [H] route of administration and [I] target therapeutic area. The chapter also includes insights into the drug developers involved in developing these products, detailing their [J] year of establishment, [K] company size, [L] location of headquarters, and [M] manufacturing facility sites.
- Key Therapeutic Areas: An extensive discussion on the most commonly targeted therapeutic indications, including [A] information on the approved / marketed injectable drug products available for the treatment of such clinical conditions (across different target therapeutic areas) and [B] their respective biosimilars.
- Likely Drug Candidates Analysis: An in-depth analysis of marketed drugs / therapies that are likely to be available in prefilled syringe format in the near future. This analysis considers various relevant parameters, including [A] historical annual sales information, [B] type of drug molecule, [C] therapeutic area, [D] route of administration and [E] other available dosage forms.
- Big Pharma Analysis: A comprehensive examination of various initiatives focused on prefilled syringe combination products undertaken by major pharmaceutical companies. This analysis includes various relevant parameters, such as [A] number of prefilled syringe combination products (approved and under development), [B] target therapeutic area and [C] type of drug molecule.
- Recent Developments: A detailed analysis of recent developments in the prefilled syringes domain, based on relevant parameters such as [A] year of initiative, [B] type of initiative (partnerships and collaborations, expansions, funding and product launches), [C] geographical distribution and [D] most active players (in terms of number of recent developments).
- Patent Analysis: Detailed analysis of various patents filed / granted related to prefilled syringes based on [A] type of patent, [B] publication year, [C] application year, [D] type of applicant, [E] geographical location, [F] CPC symbols and [G] most active players (in terms of the number of patents filed / granted). It also includes a patent benchmarking analysis and a detailed valuation analysis.
- Key Opinion Leader Analysis: An in-depth examination that emphasizes the key opinion leaders (KOLs) within this field based on several relevant parameters, such as [A] role of KOL, [B] type of sponsor organization, [C] affiliated organization, [D] target indication and [E] geographical location of KOLs. In addition, the chapter highlights the most prominent KOLs, based on our proprietary scoring criteria.
- Specialty Prefilled Syringes: Detailed information on specialty syringes, specifically prefilled flush syringes, prefilled diluent systems, and prefilled syringes with contrast agents. Each type of specialty syringe is accompanied by [A] a brief overview and [B] information on various products available in the market, along with [C] their respective advantages.
- Technological Advances Related to Prefilled Syringes: A detailed description of the recent technological advancements in this domain and their applications. The chapter highlights the advancements in design technology and manufacturing of prefilled syringes, that have enabled pharmaceutical companies to market lyophilized drugs in dual chambered syringe systems. The chapter also highlights novel lubrication and sterilization technologies.
- Key Growth Drivers: A detailed discussion on the various factors that are likely to impact the prefilled syringes market, such as [A] increase in incidence of chronic diseases, [B] rising preference for self-administration of medications, [C] evolving patient landscape, [D] introduction and [D] growing adoption of biologics / biosimilars, [E] evolving pharmaceutical strategies, [F] initiatives focused on the prevention of needlestick injuries, and [G] the various potential applications of prefilled syringes.
- SWOT Analysis: A SWOT analysis, focusing on key drivers and challenges that are likely to impact the industry's evolution. Further, it includes a Harvey ball analysis, highlighting the relative effect of each SWOT parameter on the overall industry.
- Additional Insight: The chapter discusses the growing concern associated with needlestick injuries and the various steps (including regional and global legislations) that have been taken to prevent these mishaps. In addition, it provides detailed information on the various safety features (add-on and integrated devices) installed in recent versions of prefilled syringes, and the companies engaged in development and manufacturing of such solutions.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Prefilled Syringes Market
- 1.2. Market Share Insights
- 1.2.1 Market Segmentation Overview
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. History of Prefilled Syringes
- 5.3. Benefits of Prefilled Syringes Over Traditional Injectable Devices
- 5.3.1. Benefits of Prefilled Syringes for Healthcare Professionals and End Users
- 5.3.2. Benefits of Prefilled Syringes for Manufacturers
- 5.3.3. Transition from Existing Dosing Forms to Prefilled Syringes
- 5.4. Prefilled Syringe Components
- 5.5. Classification of Prefilled Syringes
- 5.5.1. Classification based on Barrel Fabrication Material
- 5.5.1.1. Glass Barrel Prefilled Syringes
- 5.5.1.1.1. Limitations of Glass Barrel Prefilled Syringes
- 5.5.1.1.2. Addressing the Limitations of Glass Barrel Prefilled Syringes
- 5.5.1.2. Plastic Barrel Prefilled Syringes
- 5.5.1.2.1. Limitations of Plastic Barrel Prefilled Syringes
- 5.5.1.2.2. Addressing the Limitations of Plastic Barrel Prefilled Syringes
- 5.5.1.2.3. Factors Likely to Drive the Use of Plastic Prefilled Syringes
- 5.5.1.1. Glass Barrel Prefilled Syringes
- 5.5.2. Classification based on Number of Chambers in the Barrel
- 5.5.3. Classification based on Type of Needle
- 5.5.4. Classification based on Type of Packaging
- 5.5.1. Classification based on Barrel Fabrication Material
- 5.6. Critical Attributes of Prefilled Syringe Design
- 5.7. Key Manufacturing Steps for Prefilled Syringes
- 5.7.1. Production of Barrels
- 5.7.1.1. Glass Barrels
- 5.7.1.2. Plastic Barrels
- 5.7.2. Production of Syringes
- 5.7.3. Barrel Siliconization
- 5.7.4. Syringe Sterilization
- 5.7.5. Validation of Sterilization
- 5.7.6. Syringe Filling
- 5.7.7. Syringe Testing
- 5.7.1. Production of Barrels
- 5.8. Future Perspectives
6. PREFILLED SYRINGES: MARKET OVERVIEW
- 6.1. Chapter Overview
- 6.2. Prefilled Syringes: Overall Market Landscape
- 6.2.1. Analysis by Type of Barrel Fabrication Material
- 6.2.2. Analysis by Number of Barrel Chambers
- 6.2.3. Analysis by Type of Needle System
- 6.2.4. Analysis by Volume of Syringe
- 6.3. Prefilled Syringes Manufacturers: Overall Market Landscape
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters
- 6.3.4. Analysis by Company Size and Location of Headquarters
- 6.3.5. Analysis by Location of Manufacturing Facilities
- 6.3.6. Most Active Players: Analysis by Number of Prefilled Syringes
7. PRODUCT COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Assumptions and Key Parameters
- 7.3. Methodology
- 7.4. Product Competitiveness Analysis: Prefilled Syringes
- 7.4.1. Glass Barrel Prefilled Syringes
- 7.4.2. Plastic Barrel Prefilled Syringes
8. COMPANY PROFILES: PREFILLED SYRINGE MANUFACTURERS IN NORTH AMERICA
- 8.1. Chapter Overview
- 8.2. Detailed Company Profiles of Leading Prefilled Syringe Manufacturers
- 8.3.1 Becton, Dickinson and Company
- 8.3.1.1. Company Overview
- 8.3.1.2. Financial Information
- 8.3.1.3. Becton, Dickinson and Company Prefilled Syringes Portfolio
- 8.3.1.4. Recent Developments and Future Outlook
- 8.3.2. Medefil
- 8.3.2.1. Company Overview
- 8.3.2.2. Medefil Prefilled Syringes Portfolio
- 8.3.2.3. Recent Developments and Future Outlook
- 8.3.3. West Pharmaceutical
- 8.3.3.1. Company Overview
- 8.3.3.2. Financial Information
- 8.3.3.2. West Pharmaceutical Prefilled Syringes Portfolio
- 8.3.3.3. Recent Developments and Future Outlook
- 8.3.1 Becton, Dickinson and Company
- 8.4. Short Profiles of Other Prominent Prefilled Syringe Manufacturers
- 8.4.1. Medline
- 8.4.1.1. Company Overview
- 8.4.1.2. Prefilled Syringes Portfolio
- 8.4.2. MedXL
- 8.4.2.1. Company Overview
- 8.4.2.2. Prefilled Syringes Portfolio
- 8.4.3 Pfizer
- 8.4.3.1. Company Overview
- 8.4.3.2. Prefilled Syringes Portfolio
- 8.4.1. Medline
9. COMPANY PROFILES: PREFILLED SYRINGE MANUFACTURERS IN EUROPE
- 9.1. Chapter Overview
- 9.2. Detailed Company Profiles of Leading Prefilled Syringe Manufacturers
- 9.3.1. Gerresheimer
- 9.3.1.1. Company Overview
- 9.3.1.2. Financial Information
- 9.3.1.3. Gerresheimer Prefilled Syringes Portfolio
- 9.3.1.4. Recent Developments and Future Outlook
- 9.3.2 SCHOTT
- 9.3.2.1. Company Overview
- 9.3.2.2. Financial Information
- 9.3.2.3. Schott Prefilled Syringes Portfolio
- 9.3.2.4. Recent Developments and Future Outlook
- 9.3.3. Stevanato
- 9.3.3.1. Company Overview
- 9.3.2.2. Financial Information
- 9.3.3.3. Stevanato Prefilled Syringes Portfolio
- 9.3.3.4. Recent Developments and Future Outlook
- 9.3.1. Gerresheimer
- 9.4. Short Profiles of Other Prominent Prefilled Syringe Manufacturers
- 9.4.1 B. Braun
- 9.4.1.1. Company Overview
- 9.4.1.2. Prefilled Syringes Portfolio
- 9.4.2. Nipro Europe
- 9.4.2.1. Company Overview
- 9.4.2.2. Prefilled Syringes Portfolio
- 9.4.3. Vetter Pharma
- 9.4.3.1. Company Overview
- 9.4.3.2. Prefilled Syringes Portfolio
- 9.4.1 B. Braun
10. COMPANY PROFILES: PREFILLED SYRINGE MANUFACTURERS IN ASIA-PACIFIC
- 10.1. Chapter Overview
- 10.2. Detailed Company Profiles of Leading Prefilled Syringe Manufacturers
- 10.2.1 Shandong Zibo Minkang Pharmaceutical Packing
- 10.2.1.1. Company Overview
- 10.2.12. Shandong Zibo Minkang Pharmaceutical Packing Prefilled Syringes Portfolio
- 10.2.1.3. Recent Developments and Future Outlook
- 10.2.2. Shin Yan Sheno Precision Industrial
- 10.2.2.1. Company Overview
- 10.2.2.2. Taisei Kako Prefilled Syringes Portfolio
- 10.2.2.3. Recent Developments and Future Outlook
- 10.2.3. Taisei Kako
- 10.2.3.1. Company Overview
- 10.2.3.2. Taisei Kako Prefilled Syringes Portfolio
- 10.2.3.3. Recent Developments and Future Outlook
- 10.2.1 Shandong Zibo Minkang Pharmaceutical Packing
- 10.3. Short Profiles of Other Prominent Prefilled Syringe Manufacturers
- 10.3.1 J.O.Pharma
- 10.3.1.1. Company Overview
- 10.3.1.2. Prefilled Syringes Portfolio
- 10.3.2. Shandong Pharmaceutical Glass
- 10.3.2.1. Company Overview
- 10.3.2.2. Prefilled Syringes Portfolio
- 10.3.3. Shandong Weigao Group Medical Polymer
- 10.3.3.1. Company Overview
- 10.3.3.2. Prefilled Syringes Portfolio
- 10.3.1 J.O.Pharma
11. NEEDLESTICK INJURIES
- 11.1. Chapter Overview
- 11.2. Incidence and Associated Financial Burden
- 11.3. Government Legislations for Prevention of Needlestick Injuries
- 11.4. Safety Mechanisms used in Modern Prefilled Syringes
- 11.4.1. Safety Systems: Add-On Safety Device Manufacturers
- 11.4.1.1. Becton Dickinson and Company
- 11.4.1.2. Nemera
- 11.4.1.3. Nipro PharmaPackagin
- 11.4.1.4. Terumo
- 11.4.1.5. West Pharmaceutical
- 11.4.2. Safety Systems: Integrated Safety Device Manufacturers
- 11.4.2.1. Gerresheimer
- 11.4.2.2. Injecto
- 11.4.2.3. MedicalChain International
- 11.4.2.4. Stevanato
- 11.4.2.5. Owen Mumford
- 11.4.2.6. SHL Group
- 11.4.2.7. Taisei Kako
- 11.4.1. Safety Systems: Add-On Safety Device Manufacturers
12. REGULATORY LANDSCAPE FOR PREFILLED SYRINGES
- 12.1. Chapter Overview
- 12.2. Regulatory Approval of Combination Products in North America
- 12.2.1. Regulatory Approval of Combination Products in the US
- 12.2.1.1. Overview
- 12.2.1.2. Historical Background
- 12.1.1.3. Role of Regulatory Bodies in Product Approval
- 12.1.1.4. Regulatory Approval of Prefilled Syringes
- 12.1.1.4.1. Reimbursement Landscape in the US
- 12.1.1.4.2. Payer Mix
- 12.1.1.4.3.. Reimbursement Process in the US
- 12.2.2. Regulatory Approval of Combination Products in Canada
- 12.2.2.1. Overview
- 12.2.2.2. Role of Regulatory Bodies in Product Approval
- 12.2.2.3. Regulatory Approval for Prefilled Syringes
- 12.2.2.3.1. Reimbursement Landscape in Canada
- 12.2.2.3.2. Payer Mix
- 12.2.2.3.3. Reimbursement Process in Canada
- 12.2.1. Regulatory Approval of Combination Products in the US
- 12.3. Regulatory Approval of Combination Products in Europe
- 12.3.1. Overview
- 12.3.2. Role of Regulatory Bodies in Product Approval
- 12.3.3. Regulatory Approval of Prefilled Syringes
- 12.3.4. Regulatory Approval of Combination Products in the UK
- 12.3.4.1. Reimbursement Landscape in the UK
- 12.3.4.2. Payer Mix
- 12.3.4.3. Reimbursement Process in the UK
- 12.3.5. Regulatory Approval of Combination Products in France
- 12.3.5.1. Reimbursement Landscape in France
- 12.3.5.2. Payer Mix
- 12.3.5.3. Reimbursement Process in France
- 12.3.6. Regulatory Approval of Combination Products in Spain
- 12.3.6.1. Reimbursement Landscape in Spain
- 12.3.6.2. Payer Mix
- 12.3.6.3. Reimbursement Process in Spain
- 12.3.7. Regulatory Approval of Combination Products in Spain
- 12.3.7.1. Reimbursement Landscape in Germany
- 12.3.7.2. Payer Mix
- 12.3.7.3. Reimbursement Process in Germany
- 12.3.8. Regulatory Approval of Combination Products in Italy
- 12.3.8.1. Reimbursement Landscape in Italy
- 12.3.8.2. Payer Mix
- 12.3.8.3. Reimbursement Process in Italy
- 12.4. Regulatory Approval of Combination Products in Asia-Pacific
- 12.4.1. Regulatory Approval of Combination Products in Japan
- 12.4.1.1 Overview
- 12.4.1.2 Role of Regulatory Bodies in Product Approval
- 12.4.1.3. Regulatory Approval of Prefilled Syringes
- 12.4.1.3.1. Reimbursement Landscape in Japan
- 12.4.1.3.2. Payer Mix
- 12.4.1.3.3. Reimbursement Process in Japan
- 12.4.2. Regulatory Approval of Combination Products in China
- 12.4.2.1. Overview
- 12.4.2.2. Role of Regulatory Bodies in Product Approval
- 12.4.2.3. Regulatory Approval for Prefilled syringes
- 12.4.2.3.1. Reimbursement Landscape in China
- 12.4.2.3.2. Payer Mix
- 12.4.2.3.3. Reimbursement Process in China
- 12.4.3. Regulatory Approval of Combination Products in India
- 12.4.3.1. Overview
- 12.4.3.2. Role of Regulatory Bodies in Product Approval
- 12.4.3.3. Regulatory Approval of Prefilled Syringes
- 12.4.3.3.1. Reimbursement Landscape in India
- 12.4.3.3.2. Payer Mix
- 12.4.4. Regulatory Approval of Combination Products in South Korea
- 12.4.4.1. Overview
- 12.4.4.2. Role of Regulatory Bodies in Product Approval
- 12.4.4.3. Regulatory Approval of Prefilled Syringes
- 12.4.4.3.1. Reimbursement Landscape in South Korea
- 12.4.4.3.2. Payer Mix
- 12.4.4.3.3. Reimbursement Process in South Korea
- 12.4.5. Regulatory Approval of Combination Products in Saudi Arabia
- 12.4.5.1. Overview
- 12.4.5.2. Role of Regulatory Bodies in Product Approval
- 12.4.5.3. Regulatory Approval of Prefilled syringes
- 12.4.5.3.1. Reimbursement Landscape in Saudi Arabia
- 12.4.5.3.2. Payer Mix
- 12.4.5.3.3. Reimbursement Process in Saudi Arabia
- 12.4.6. Regulatory Approval of Combination Products in United Arab Emirates
- 12.4.6.1. Overview
- 12.4.6.2. Role of Regulatory Bodies in Product Approval
- 12.4.6.3. Regulatory Approval of Prefilled syringes
- 12.4.6.3.1. Reimbursement Landscape in United Arab Emirates
- 12.4.6.3.2. Payer Mix
- 12.4.6.3.3. Reimbursement Process in United Arab Emirates
- 12.4.1. Regulatory Approval of Combination Products in Japan
- 12.5. Regulatory Approval of Combination Products in Rest of the World
- 12.5.1. Regulatory Approval of Combination Products in Brazil
- 12.5.1.1 Overview
- 12.5.1.2 Role of Regulatory Bodies in Product Approval
- 12.5.1.3. Regulatory Approval of Prefilled Syringes
- 12.5.1.3.1. Reimbursement Landscape in Brazil
- 12.5.1.3.2. Payer Mix
- 12.5.1.3.3. Reimbursement Process in Brazil
- 12.5.2. Regulatory Approval of Combination Products in Australia
- 12.5.2.1 Overview
- 12.5.2.2 Role of Regulatory Bodies in Product Approval
- 12.5.2.3. Regulatory Approval of Prefilled Syringes
- 12.5.2.3.1. Reimbursement Landscape in Australia
- 12.5.2.3.2. Payer Mix
- 12.5.2.3.3. Reimbursement Process in Australia
- 12.5.1. Regulatory Approval of Combination Products in Brazil
13. PREFILLED SYRINGE COMBINATION PRODUCTS
- 13.1. Chapter Overview
- 13.2. Prefilled Syringe Combination Products: Approved Drugs
- 13.2.1. Analysis by Type of Drug Molecule
- 13.2.2. Analysis by Product Approval
- 13.2.3. Analysis by Route of Administration
- 13.2.4. Analysis by Target Therapeutic Area
- 13.2.5. Analysis by Region of Product Analysis
- 13.2.6. Analysis by Other Approved Primary Packaging Systems
- 13.3. Prefilled Syringe Combination Products: Clinical Stage Drugs
- 13.3.1. Analysis by Type of Drug Molecule
- 13.3.2. Analysis by Phase of Development
- 13.3.3. Analysis by Route of Administration
- 13.3.4. Analysis by Target Therapeutic Area
- 13.4. Prefilled Syringe Combination Products: Developers Landscape
- 13.4.1. Analysis by Year of Establishment
- 13.4.2. Analysis by Company Size
- 13.4.3. Analysis by Location of Headquarters
- 13.5. Leading Drugs in Prefilled Syringe Format
- 13.6. Other Drugs Available in Prefilled Syringe Format
- 13.7. Most Popular Drug Available in Prefilled Syringe Format: Humira (adalimumab), AbbVie / Eisai
- 13.7.1. Target Indications and Available Dosage Forms
- 13.7.2. Shift from Vials to Prefilled Syringes
- 13.7.3. Humira Annual Sales, FY 2010-FY 2022
14. KEY THERAPEUTIC AREAS
- 14.1. Chapter Overview
- 14.2. Autoimmune Disorders
- 14.2.1. Approved Drugs
- 14.2.2. Key Developments
- 14.2.3. Upcoming / Commercialized Biosimilars for Autoimmune Drugs
- 14.3. Infectious Diseases
- 14.3.1. Approved Drugs
- 14.3.2. Key Developments
- 14.4. Neurological Disorders
- 14.4.1. Approved Drugs
- 14.4.2. Key Developments
15. PREFILLED SYRINGES: LIKELY DRUG CANDIDATES ANALYSIS
- 15.1. Chapter Overview
- 15.2. Likely Drug Candidates
- 15.2.1. Assumption and Key Parameters
- 15.2.2. Methodology
- 15.2.3. Most Likely Candidates for Delivery via Prefilled Syringes
- 15.2.4. Likely Candidates for Delivery via Prefilled Syringes
- 15.2.5. Less Likely Candidates for Delivery Via Prefilled Syringe
16. COMBINATION PRODUCTS: BIG PHARMA ACTIVITY
- 16.1. Chapter Overview
- 16.2. Methodology
- 16.3. Combination Products: Big Pharma Activity
- 16.3.1. Analysis by Target Therapeutic Area
- 16.3.1.1. Autoimmune Disorders
- 16.3.1.2. Infectious Diseases
- 16.3.1.3. Neurological Disorders
- 16.3.1.4. Blood Disorders
- 16.3.1.5. Oncological Disorders
- 16.3.2. Analysis by Type of Molecule
- 16.3.2.1. Antibodies
- 16.3.2.2. Vaccines
- 16.3.2.3. Proteins
- 16.3.2.4. Peptides
- 16.3.2.5. Small Molecules
- 16.3.2.6. Other Biologics
- 16.3.1. Analysis by Target Therapeutic Area
17. RECENT DEVELOPMENTS
- 17.1. Chapter Overview
- 17.2. Prefilled Syringes: Recent Developments
- 17.3. Analysis by Year of Initiative
- 17.4. Analysis by Type of Initiative
- 17.5. Analysis by Year and Type of Initiative
- 17.5.1. Analysis by Location of Expansion
- 17.5.2. Analysis by Type of Expansion
- 17.5.3 Analysis by Type of Partnership
- 17.6. Analysis by Geography
- 17.7. Most Active Players: Distribution by Number of Recent Initiatives
- 17.8. Analysis of Player by Type of Initiatives
18. PATENT ANALYSIS
- 18.1. Chapter Overview
- 18.2. Scope and Methodology
- 18.3. Prefilled Syringes: Patent Analysis
- 18.3.1. Analysis by Patent Publication Year
- 18.3.2. Analysis by Type of Patent and Publication Year
- 18.3.3. Analysis by Patent Application Year
- 18.3.4. Analysis by Patent Jurisdiction
- 18.3.5. Analysis by CPC Symbols
- 18.3.6. Analysis by Type of Applicant
- 18.3.7. Leading Players: Analysis by Number of Patents
- 18.3.8. Leading Individual Assignees: Analysis by Number of Patents
- 18.4. Prefilled Syringes: Patent Benchmarking Analysis
- 18.4.1. Analysis by Patent Characteristics
- 18.5. Prefilled Syringes: Patent Valuation Analysis
- 18.6. Leading Patents by Number of Citations
19. KOL ANALYSIS
- 19.1. Chapter Overview
- 19.2. Assumption and Key Parameters
- 19.3. Methodology
- 19.4. Prefilled Syringes: Key Opinion Leaders
- 19.4.1. Analysis by Role of KOL
- 19.4.2. Analysis by Type of Sponsor Organization
- 19.4.3. Analysis by Affiliated Organization
- 19.4.4. Analysis by Target Disease Indication
- 19.4.5. Analysis by Geographical Location
- 19.4.6. Most Prominent KOLs: Analysis by Activeness, Expertise and Strength of KOL
- 19.4.7. Most Prominent KOLs: Analysis by RA score
20. SPECIALTY PREFILLED SYRINGES
- 20.1. Chapter Overview
- 20.2. Prefilled Flush Syringes
- 20.2.1. Overview
- 20.2.2. Advantages of Prefilled Flush Syringes
- 20.2.3. Prefilled Flush Syringes Available in the Market
- 20.3. Prefilled Diluent Syringes
- 20.3.1. Overview
- 20.3.2. Advantages of Prefilled Diluent Syringes
- 20.3.3. Lyophilized Drugs Available in Prefilled Diluent Syringes
- 20.4. Contrast Agent Prefilled Syringes
- 20.4.1. Overview
- 20.4.2. Advantages of Using Prefilled Syringes for Contrast Agents
- 20.4.3. Contrast Agents Available in Prefilled Syringes
21. TECHNOLOGICAL ADVANCES RELATED TO PREFILLED SYRINGES
- 21.1. Chapter Overview
- 21.2. Prefilled Syringes for Lyophilized Drugs
- 21.2.1. Prefilled Diluent Syringes
- 21.2.2. Prefilled Dual-Chamber Syringes
- 21.3. Multilayer Plastic Prefilled Syringes with Oxygen Barrier
- 21.4. Prefilled Syringes with Low Risk of Particle Formation
- 21.5. Advanced Lubrication Technology for Prefilled Syringes
- 21.6. Advances in Terminal Sterilization of Prefilled Syringes
- 21.7. Prefilled Syringe Usage Aids for Patients and Healthcare Providers
- 21.8. Additional Advancements
22. KEY GROWTH DRIVERS
- 22.1. Chapter Overview
- 22.2. Rising Incidence of Chronic Diseases
- 22.3. Growing Preference for Self-Injection
- 22.4. Evolving Patient Demographics
- 22.5. Growth of Biologics and Biosimilars Market
- 22.6. Changing Pharmaceutical Practices
- 22.7. Increasing Focus on Prevention of Needlestick Injuries
- 22.8. Prefilled Syringes in Autoinjectors and Pen Injectors
23. SWOT ANALYSIS
- 23.1. Chapter Overview
- 23.2. Strengths
- 23.2.1. Ease of Use
- 23.2.2. Ability to Reduce / Eliminate Medication Errors
- 23.2.3. Reducing the Risk of Accidental Transmission of Blood-Borne Pathogens
- 23.2.4. Economic Advantages
- 23.2.5. Drug Life Cycle Management
- 23.3. Weaknesses
- 23.3.1. Manufacturing Complexities
- 23.3.2. High Packaging and Transportation Costs
- 23.3.3. Product Stability and Quality Related Issues
- 23.3.4. Challenges Associated with Drug Filing
- 23.4. Opportunities
- 23.4.1. Rich and Growing Pipeline of Biologics and Biosimilars
- 23.4.2. Growing Preference for Self-Injecting
- 23.4.3. Application in Advanced Drug Delivery Devices
- 23.4.4. Innovation in Design and Technical Advancement
- 23.4.5. Rising Popularity across the Globe
- 23.4.6. Widespread Use in Vaccination Programs
- 23.5. Threats
- 23.5.1. Material Compatibility Issues
- 23.5.2. Availability of Alternative Drug Delivery Devices
- 23.5.3. Concern Related to Numerous Product Recalls In Past
- 23.6. Concluding Remarks
24. GLOBAL PREFILLED SYRINGES MARKET
- 24.1. Chapter Overview
- 24.2. Assumptions and Methodology
- 24.3. Global Prefilled Syringes Market, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 24.3.1. Scenario Analysis
- 24.4. Key Market Segmentations
25. PREFILLED SYRINGES MARKET, BY PURPOSE OF SYRINGE
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Prefilled Syringes Market: Distribution by Purpose of Syringe, 2018, 2023 and 2035
- 25.3.1. Specialty Prefilled Syringe: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 25.3.2. Therapeutic Prefilled Syringe: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 25.3.3. Cosmetic Prefilled Syringe: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 25.4. Data Triangulation and Validation
26. PREFILLED SYRINGES MARKET, BY TYPE OF MOLECULE
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Prefilled Syringes Market: Distribution by Type of Molecule, 2018, 2023 and 2035
- 26.3.1. Prefilled Syringes Market for Proteins: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.3.2. Prefilled Syringes Market for Antibodies: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.3.3. Prefilled Syringes Market for Peptides: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.3.4. Prefilled Syringes Market for Small Molecules: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.3.5. Prefilled Syringes Market for Vaccines: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.3.6. Prefilled Syringes Market for Cell Therapies: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 26.4. Data Triangulation and Validation
27. PREFILLED SYRINGES MARKET, BY THERAPEUTIC AREA
- 27.1. Chapter Overview
- 27.2. Key Assumptions and Methodology
- 27.3. Prefilled Syringes Market: Distribution by Therapeutic Area, 2018, 2023 and 2035
- 27.3.1. Prefilled Syringes Market for Blood Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.2. Prefilled Syringes Market for Infectious Diseases: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.3. Prefilled Syringes Market for Autoimmune Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.4. Prefilled Syringes Market for Oncological Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.5. Prefilled Syringes Market for Cardiovascular Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.6. Prefilled Syringes Market for Respiratory Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.7. Prefilled Syringes Market for Neurological Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.8. Prefilled Syringes Market for Metabolic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.9. Prefilled Syringes Market for Ophthalmic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.10. Prefilled Syringes Market for Orthopedic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.3.11 Prefilled Syringes Market for Other Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 27.4. Data Triangulation and Validation
28. PREFILLED SYRINGES MARKET, BY TYPE OF BARREL MATERIAL
- 28.1. Chapter Overview
- 28.2. Key Assumptions and Methodology
- 28.3. Prefilled Syringes Market: Distribution by Type of Barrel Material, 2018, 2023 and 2035
- 28.3.1. Glass Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 28.3.2. Plastic Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 28.4. Data Triangulation and Validation
29. PREFILLED SYRINGES MARKET, BY NUMBER OF CHAMBER
- 29.1. Chapter Overview
- 29.2. Key Assumptions and Methodology
- 29.3. Prefilled Syringes Market: Distribution by Number of Chamber, 2018, 2023 and 2035
- 29.3.1. Single Chamber Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 29.3.2. Dual Chamber Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 29.4. Data Triangulation and Validation
30. PREFILLED SYRINGES MARKET, BY TYPE OF NEEDLE SYSTEM
- 30.1. Chapter Overview
- 30.2. Key Assumptions and Methodology
- 30.3. Prefilled Syringes Market: Distribution by Type of Needle System, 2018, 2023 and 2035
- 30.3.1. Staked Needle Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 30.3.2. Luer Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 30.4. Data Triangulation and Validation
31. PREFILLED SYRINGES MARKET, BY USABILITY OF SYRINGE
- 31.1. Chapter Overview
- 31.2. Key Assumptions and Methodology
- 31.3. Prefilled Syringes Market: Distribution by Usability of Syringe, 2018, 2023 and 2035
- 31.3.1. Disposable Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 31.3.2. Reusable Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 31.4. Data Triangulation and Validation
32. PREFILLED SYRINGES MARKET, BY TYPE OF SYRINGE
- 32.1. Chapter Overview
- 32.2. Key Assumptions and Methodology
- 32.3. Prefilled Syringes Market: Distribution by Type of Syringe, 2018, 2023 and 2035
- 32.3.1. Conventional Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 32.3.1. Safety Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 32.4. Data Triangulation and Validation
33. PREFILLED SYRINGES MARKET, BY TYPE OF PACKAGING
- 33.1. Chapter Overview
- 33.2. Key Assumptions and Methodology
- 33.3. Prefilled Syringes Market: Distribution by Type of Packaging, 2018, 2023 and 2035
- 33.3.1. Bulk Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 33.3.2. Nested Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 33.4. Data Triangulation and Validation
34. PREFILLED SYRINGES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 34.1. Chapter Overview
- 34.2. Key Assumptions and Methodology
- 34.3. Prefilled Syringes Market: Distribution by Key Geographical Region, 2018, 2023 and 2035
- 34.3.1 Prefilled Syringes Market in North America: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 34.3.2. Prefilled Syringes Market in Europe: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 34.3.3. Prefilled Syringes Market in Asia-Pacific: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 34.3.4. Prefilled Syringes Market in Middle East and South Africa: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 34.3.5 Prefilled Syringes Market in Latin America: Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 34.4. Data Triangulation and Validation
35. PREFILLED SYRINGES MARKET, FOR BLOOD DISORDERS
- 35.1. Chapter Overview
- 35.2. Key Assumptions and Methodology
- 35.3. Prefilled Syringes Market for Blood Disorders: Distribution by Type of Molecule
- 35.3.1. Proteins Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.3.2. Small Molecules Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.4. Prefilled Syringes Market for Blood Disorders: Distribution by Type of Barrel Material
- 35.4.1. Glass Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.4.2. Plastic Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.5. Prefilled Syringes Market for Blood Disorders: Distribution by Number of Chamber
- 35.5.1. Single Chamber Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.5.2. Dual Chamber Prefilled Syringes, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.6. Prefilled Syringes Market for Blood Disorders: Distribution by Type of Needle System
- 35.6.1. Staked Needle Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.6.2. Luer Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.7. Prefilled Syringes Market for Blood Disorders: Distribution by Usability of Syringe
- 35.7.1. Disposable Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.7.2. Reusable Prefilled Syringes Market For Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.8. Prefilled Syringes Market for Blood Disorders: Distribution by Type of Syringe
- 35.8.1. Conventional Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.8.2. Safety Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.9. Prefilled Syringes Market for Blood Disorders: Distribution by Type of Packaging
- 35.9.1. Bulk Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.9.2. Nested Prefilled Syringes Market for Blood Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 35.10. Prefilled Syringes Market for Blood Disorders: Distribution by Key Geographical Region
- 35.10.1. Prefilled Syringes Market for Blood Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.10.2. Prefilled Syringes Market for Blood Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.10.3. Prefilled Syringes Market for Blood Disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.10.4. Prefilled Syringes Market for Blood Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 35.10.5. Prefilled Syringes Market for Blood Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
36. PREFILLED SYRINGES MARKET, FOR INFECTIOUS DISEASES
36. Prefilled Syringes Market for Infectious Diseases
- 36.1. Chapter Overview
- 36.2. Key Assumptions and Methodology
- 36.3. Prefilled Syringes Market for Infectious Diseases: Distribution by Type of Molecule
- 36.3.1. Proteins Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.3.2. Vaccines Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.4. Prefilled Syringes Market for Infectious Diseases: Distribution by Type of Barrel Material
- 36.4.1. Glass Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.4.2. Plastic Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.5. Prefilled Syringes Market for Infectious Diseases: Distribution by Number of Chamber
- 36.5.1. Single Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.5.2. Dual Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.6. Prefilled Syringes Market for Infectious Diseases: Distribution by Type of Needle System
- 36.6.1. Staked Needle Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.6.2. Luer Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.7. Prefilled Syringes Market for Infectious Diseases: Distribution by Usability of Syringe
- 36.7.1. Disposable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.7.2. Reusable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.8. Prefilled Syringes Market for Infectious Diseases: Distribution by Type of Syringe
- 36.8.1. Conventional Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.8.2. Safety Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.9. Prefilled Syringes Market for Infectious Diseases: Distribution by Type of Packaging
- 36.9.1. Bulk Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.9.2. Nested Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.10. Prefilled Syringes Market for Infectious Diseases: Distribution by Key Geographical Region
- 36.10.1. Prefilled Syringes Market for Infectious Diseases in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.10.2. Prefilled Syringes Market for Infectious Diseases in Europe, Historical Trends (since 2018) and Forecasted Estimates(till 2035)
- 36.10.3. Prefilled Syringes Market for Infectious Diseases in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.10.4. Prefilled Syringes Market for Infectious Diseases in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 36.10.5. Prefilled Syringes Market for Infectious Diseases in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
37. PREFILLED SYRINGES MARKET, FOR AUTOIMMUNE DISORDERS
- 37.1. Chapter Overview
- 37.2. Key Assumptions and Methodology
- 37.3. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Type of Molecule
- 37.3.1. Proteins Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.3.2. Antibodies Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.3.3. Peptides Market for Autoimmune Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035)
- 37.3.4. Small Molecule Market for Autoimmune Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035)
- 37.4. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Type of Barrel Material
- 37.4.1. Glass Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.4.2. Plastic Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.5. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Number of Chamber
- 37.5.1. Single Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.5.2. Dual Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.6. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Type of Needle System
- 37.6.1. Staked Needle Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.6.2. Luer Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.7. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Usability of Syringe
- 37.7.1. Disposable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.7.2. Reusable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.8. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Type of Syringe
- 37.8.1. Conventional Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.8.2. Safety Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 37.9. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Type of Packaging
- 37.9.1. Bulk Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.9.2. Nested Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 37.10. Prefilled Syringe Market for Autoimmune Disorders: Distribution by Key Geographical Region
- 37.10.1. Prefilled Syringes Market for Autoimmune Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.10.2. Prefilled Syringes Market for Autoimmune Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.10.3. Prefilled Syringes Market for Autoimmune Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.10.4. Prefilled Syringes Market for Autoimmune Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 37.10.5. Prefilled Syringes Market for Autoimmune Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
38. PREFILLED SYRINGES MARKET, FOR ONCOLOGICAL DISORDERS
- 38.1. Chapter Overview
- 38.2. Key Assumptions and Methodology
- 38.3. Prefilled Syringe Market for Oncological disorders: Distribution by Type of Molecule
- 38.3.1. Proteins Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.3.2. Antibodies Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.3.3. Peptides Market for Oncological Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035)
- 38.3.4. Small Molecules Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.3.5. Vaccines Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.4. Prefilled Syringe Market for Oncological disorders: Distribution by Type of Barrel Material
- 38.4.1. Glass Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.4.2. Plastic Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.5. Prefilled Syringe Market for Oncological disorders: Distribution by Number of Chamber
- 38.5.1. Single Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.5.2. Dual Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.6. Prefilled Syringe Market for Oncological disorders: Distribution by Type of Needle System
- 38.6.1. Staked Needle Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.6.2. Luer Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.7. Prefilled Syringe Market for Oncological disorders: Distribution by Usability of Syringe
- 38.7.1. Disposable Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.7.2. Reusable Barrel Prefilled Syringes, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.8. Prefilled Syringe Market for Oncological Disorders: Distribution by Type of Syringe
- 38.8.1. Conventional Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.8.2. Safety Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.9. Prefilled Syringe Market for Oncological disorders: Distribution by Type of Packaging
- 38.9.1. Bulk Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.9.2. Nested Prefilled Syringes Market for Oncological Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 38.10. Prefilled Syringes Market for Oncological disorders: Distribution by Key Geographical Regions
- 38.10.1. Prefilled Syringes Market for Oncological disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.10.2. Prefilled Syringes Market for Oncological disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.10.3. Prefilled Syringes Market for Oncological Disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.10.4. Prefilled Syringes Market for Oncological Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 38.10.5. Prefilled Syringes Market for Oncological Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
39. PREFILLED SYRINGES MARKET, FOR CARDIOVASCULAR DISORDERS
- 39.1. Chapter Overview
- 39.2. Key Assumptions and Methodology
- 39.3. Prefilled Syringes Market for Cardiovascular Disorders: Distribution by Type of Molecule
- 39.3.1. Antibodies Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.3.2. Cell Therapies Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.4. Prefilled Syringes Market for Cardiovascular disorders: Distribution by Type of Barrel Material
- 39.4.1. Glass Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.4.2. Plastic Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.5. Prefilled Syringes Market for Cardiovascular disorders: Distribution by Number of Chamber
- 39.5.1. Single Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.5.2. Dual Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.6. Prefilled Syringes Market for Cardiovascular disorders: Distribution by Type of Needle System
- 39.6.1. Staked Needle Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.6.2. Luer Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.7. Prefilled Syringes Market for Cardiovascular disorders: Distribution by Usability of Syringe
- 39.7.1. Disposable Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.7.2. Reusable Barrel Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.8. Prefilled Syringes Market for Cardiovascular disorders: Distribution by Type of Syringe
- 39.8.1. Conventional Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.8.2. Safety Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.9. Prefilled Syringes Market for Cardiovascular Disorders: Distribution by Type of Packaging
- 39.9.1. Bulk Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.9.2. Nested Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 39.10. Prefilled Syringes Market for Cardiovascular Disorders: Distribution by Key Geographical Regions
- 39.10.1. Prefilled Syringes Market for Cardiovascular Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.10.2. Prefilled Syringes Market for Cardiovascular Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.10.3. Prefilled Syringes Market for Cardiovascular Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.10.4. Prefilled Syringes Market for Cardiovascular Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 39.10.5. Prefilled Syringes Market for Cardiovascular Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
40. PREFILLED SYRINGES MARKET, FOR RESPIRATORY DISORDERS
- 40.1. Chapter Overview
- 40.2. Key Assumptions and Methodology
- 40.3. Prefilled Syringes Market for Respiratory disorders: Distribution by Type of Molecule
- 40.3.1. Antibodies Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.4. Prefilled Syringes Market for Respiratory disorders: Distribution by Type of Barrel Material
- 40.4.1. Glass Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.4.2. Plastic Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.5. Prefilled Syringes Market for Respiratory disorders: Distribution by Number of Chamber
- 40.5.1. Single Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.5.2. Dual Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.6. Prefilled Syringes Market for Respiratory disorders: Distribution by Type of Needle System
- 40.6.1. Staked Needle Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.6.2. Luer Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.7. Prefilled Syringes Market for Respiratory Disorders: Distribution by Usability of Syringe
- 40.7.1. Disposable Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.7.2. Reusable Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.8. Prefilled Syringes Market for Respiratory Disorders: Distribution by Type of Syringe
- 40.8.1. Conventional Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.8.2. Safety Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.9. Prefilled Syringes Market for Respiratory Disorders: Distribution by Type of Packaging
- 40.9.1. Bulk Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.9.2. Nested Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.10. Prefilled Syringes Market for Respiratory Disorders: Distribution by Key Geographical Regions
- 40.10.1. Prefilled Syringes Market for Respiratory Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.10.2. Prefilled Syringes Market for Respiratory Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.10.3. Prefilled Syringes Market for Respiratory Disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.10.4. Prefilled Syringes Market for Respiratory Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 40.10.5. Prefilled Syringes Market for Respiratory Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
41. PREFILLED SYRINGES MARKET, FOR NEUROLOGICAL DISORDERS
- 41.1. Chapter Overview
- 41.2. Key Assumptions and Methodology
- 41.3. Prefilled Syringes Market for Neurological Disorders: Distribution by Type of Molecule
- 41.3.1. Antibodies Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.3.2. Proteins Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.3.3. Peptides Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.3.4. Small Molecules Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.4. Prefilled Syringes Market for Neurological Disorders: Distribution by Type of Barrel Material
- 41.4.1. Glass Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.4.2. Plastic Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.5. Prefilled Syringes Market for Neurological Disorders: Distribution by Number of Chamber
- 41.5.1. Single Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.5.2. Dual Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.6. Prefilled Syringes Market for Neurological Disorders: Distribution by Type of Needle System
- 41.6.1. Staked Needle Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.6.2. Luer Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.7. Prefilled Syringes Market for Neurological Disorders: Distribution by Usability of Syringe
- 41.7.1. Disposable Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.7.2. Reusable Barrel Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.8. Prefilled Syringes Market for Neurological Disorders: Distribution by Type of Syringe
- 41.8.1. Conventional Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.8.2. Safety Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.9. Prefilled Syringes Market for Neurological Disorders: Distribution by Type of Packaging
- 41.9.1. Bulk Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.9.2. Nested Prefilled Syringes Market for Neurological Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 41.10. Prefilled Syringes Market for Neurological Disorders: Distribution by Key Geographical Regions
- 41.10.1. Prefilled Syringes Market for Neurological Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.10.2. Prefilled Syringes Market for Neurological Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.10.3. Prefilled Syringes Market for Neurological Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.10.4. Prefilled Syringes Market for Neurological Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 41.10.5. Prefilled Syringes Market for Neurological Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
42. PREFILLED SYRINGES MARKET, FOR METABOLIC DISORDERS
- 42.1. Chapter Overview
- 42.2. Key Assumptions and Methodology
- 42.3. Prefilled Syringes Market for Metabolic Disorders: Distribution by Type of Molecule
- 42.3.1. Proteins Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.3.2. Small Molecule Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.3.3. Peptides Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.4. Prefilled Syringes Market for Metabolic disorders: Distribution by Type of Barrel Material
- 42.4.1. Glass Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.4.2. Plastic Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.5. Prefilled Syringes Market for Metabolic Disorders: Distribution by Number of Chamber
- 42.5.1. Single Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.5.2. Dual Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.6. Prefilled Syringes Market for Metabolic Disorders: Distribution by Type of Needle System
- 42.6.1. Staked Needle Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.6.2. Luer Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.7. Prefilled Syringes Market for Metabolic Disorders: Distribution by Usability of Syringe
- 42.7.1. Disposable Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.7.2. Reusable Barrel Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.8. Prefilled Syringes Market for Metabolic Disorders: Distribution by Type of Syringe
- 42.8.1. Conventional Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.8.2. Safety Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.9. Prefilled Syringes Market for Metabolic Disorders: Distribution by Type of Packaging
- 42.9.1. Bulk Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.9.2. Nested Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.10. Prefilled Syringes Market for Metabolic Disorders: Distribution by Key Geographical Regions
- 42.10.1. Prefilled Syringes Market for Metabolic Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.10.2. Prefilled Syringes Market for Metabolic Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.10.3. Prefilled Syringes Market for Metabolic disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.10.4. Prefilled Syringes Market for Metabolic disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 42.10.5. Prefilled Syringes Market for Metabolic disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
43. PREFILLED SYRINGES MARKET, FOR OPHTHALMIC DISORDERS
- 43.1. Chapter Overview
- 43.2. Key Assumptions and Methodology
- 43.3. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Type of Molecule
- 43.3.1. Proteins Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.3.2. Antibodies for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.4. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Type of Barrel Material
- 43.4.1. Glass Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.4.2. Plastic Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.5. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Number of Chamber
- 43.5.1. Single Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.5.2. Dual Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.6. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Type of Needle System
- 43.6.1. Staked Needle Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.6.2. Luer Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.7. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Usability of Syringe
- 43.7.1. Disposable Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.7.2. Reusable Barrel Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.8. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Type of Syringe
- 43.8.1. Conventional Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.8.2. Safety Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.9. Prefilled Syringes Market for Ophthalmic Disorders: Distribution by Type of Packaging
- 43.9.1. Bulk Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.9.2. Nested Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 43.10. Prefilled Syringes Market for Ophthalmic disorders: Distribution by Key Geographical Regions
- 43.10.1. Prefilled Syringes Market for Ophthalmic disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.10.2. Prefilled Syringes Market for Ophthalmic disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.10.3. Prefilled Syringes Market for Ophthalmic disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.10.4. Prefilled Syringes Market for Ophthalmic disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 43.10.5. Prefilled Syringes Market for Ophthalmic disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
44. PREFILLED SYRINGES MARKET, FOR ORTHOPEDIC DISORDERS
- 44.1. Chapter Overview
- 44.2. Key Assumptions and Methodology
- 44.3. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Type of Molecule
- 44.3.1. Antibodies Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.3.2. Small Molecules Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.3.3. Cell Therapies Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.4. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Type of Barrel Material
- 44.4.1. Glass Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.4.2. Plastic Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.5. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Number of Chamber
- 44.5.1. Single Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.5.2. Dual Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.6. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Type of Needle System
- 44.6.1. Staked Needle Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.6.2. Luer Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.7. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Usability of Syringe
- 44.7.1. Disposable Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.7.2. Reusable Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.8. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Type of Syringe
- 44.8.1. Conventional Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.8.2. Safety Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.9. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Type of Packaging
- 44.9.1. Bulk Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.9.2. Nested Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (2018- 2022) and Forecasted Estimates (till 2035)
- 44.10. Prefilled Syringes Market for Orthopedic Disorders: Distribution by Key Geographical Regions
- 44.10.1. Prefilled Syringes Market for Orthopedic disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.10.2. Prefilled Syringes Market for Orthopedic disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.10.3. Prefilled Syringes Market for Orthopedic disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.10.4. Prefilled Syringes Market for Orthopedic disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 44.10.5. Prefilled Syringes Market for Orthopedic disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
45. PREFILLED SYRINGES MARKET, FOR OTHER DISORDERS
- 45.1. Chapter Overview
- 45.2. Key Assumptions and Methodology
- 45.3. Prefilled Syringes Market for Other Disorders: Distribution by Type of Molecule
- 45.3.1. Proteins Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.3.2. Antibodies Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.3.3. Peptides Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.3.4. Small Molecules Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.3.5. Vaccines Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.3.6. Cell Therapies Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.4. Prefilled Syringes Market for Other Disorders: Distribution by Type of Barrel Material
- 45.4.1. Glass Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.4.2. Plastic Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.5. Prefilled Syringes Market for Other Disorders: Distribution by Number of Chamber
- 45.5.1. Single Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.5.2. Dual Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.6. Prefilled Syringes Market for Other Disorders: Distribution by Type of Needle System
- 45.6.1. Staked Needle Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.6.2. Luer Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.7. Prefilled Syringes Market for Other Disorders: Distribution by Usability of Syringe
- 45.7.1. Disposable Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.7.2. Reusable Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.8. Prefilled Syringes Market for Other Disorders: Distribution by Type of Syringe
- 45.8.1. Conventional Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.8.2. Safety Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.9. Prefilled Syringes Market for Other Disorders: Distribution by Type of Packaging
- 45.9.1. Bulk Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.9.2. Nested Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.10. Prefilled Syringes Market for Other Disorders: Distribution by Key Geographical Regions
- 45.10.1. Prefilled Syringes Market for Other disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.10.2. Prefilled Syringes Market for Other disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.10.3. Prefilled Syringes Market for Other disorders in Asia- Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.10.4. Prefilled Syringes Market for Other disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
- 45.10.5. Prefilled Syringes Market for Other disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035)
46. PREFILLED SYRINGE COMPONENT MANUFACTURERS
- 46.1. Chapter Overview
- 46.2. List of Component Manufacturers
- 46.3. Prominent Prefilled Syringe Component Manufacturers: Companies Profiled
- 46.3.1. West Pharmaceutical Services
- 46.3.1.1. Company Overview
- 46.3.1.2. Financial Information
- 46.3.1.3. Product Portfolio
- 46.3.1.4. Recent Developments and Future Outlook
- 46.3.2. Datwyler
- 46.3.2.1. Company Overview
- 46.3.2.2. Financial Information
- 46.3.2.3. Product Portfolio
- 46.3.2.4. Recent Developments and Future Outlook
- 46.3.3. Aptar Pharma
- 46.3.3.1. Company Overview
- 46.3.3.2. Financial Information
- 46.3.3.3. Product Portfolio
- 46.3.3.4. Recent Developments and Future Outlook
- 46.3.4. Stevanato
- 46.3.4.1. Company Overview
- 46.3.4.2. Financial Information
- 46.3.4.3. Product Portfolio
- 46.3.4.4. Recent Developments and Future Outlook
- 46.3.1. West Pharmaceutical Services
47. FILL / FINISH SERVICE PROVIDERS FOR PREFILLED SYRINGES
- 47.1. Chapter Overview
- 47.2. Fill / Finish Processing of Prefilled Syringes
- 47.2.1. Steps Involved in Fill / Finish Process
- 47.2.2. Methods of Filling and Stoppering of Prefilled Syringes
- 47.2.3. Prefilled Syringe Filling Technologies
- 47.3. Outsourcing of Fill / Finish Operations
- 47.4. Growth Considerations
- 47.4.1. Recent Developments
- 47.5. Prefilled Syringes: Fill / Finish Service Providers
- 47.5.1. Analysis by Year of Establishment
- 47.5.2. Analysis by Location of Headquarters and Type of Molecule Filled
- 47.5.3. Analysis by Scale of Operation
48. CASE STUDY: AUTOINJECTORS
- 48.1. Chapter Overview
- 48.2. Autoinjectors Compatible with Prefilled Syringes
- 48.3. Key Players
- 48.3.1. Owen Mumford
- 48.3.1.1. Company Overview
- 48.3.1.2. Product Portfolio
- 48.3.1.2.1 Autoinjectors: Product Details
- 48.3.1.2.1.1 Autoinjectors Micro
- 48.3.1.2.1.2. Autoject 2
- 48.3.1.2.1.3. Autoject Mini
- 48.3.1.2.1.4. Autoject Visco
- 48.3.1.2.1.5. Autoject Uni
- 48.3.1.2.1.6. Autoject Multi
- 48.3.1.2.1.7. Autoject Flex
- 48.3.1.2.1.8. Unisafe Auto-injector
- 48.3.1.2.1.9. Unisafe Connected Auto-injector
- 48.3.1.2.1.10. Aidaptus
- 48.3.1.2.1 Autoinjectors: Product Details
- 48.3.2. Elcam Medical (E3D Elcam Drug Delivery Devices)
- 48.3.2.1. Company Overview
- 48.3.2.2. Product Portfolio
- 48.3.2.2.1 Autoinjectors: Product Details
- 48.3.2.2.1.1. Flexi-Q Disposable Autoinjectors
- 48.3.2.2.1.2. Flexi-Q Reusable Autoinjectors
- 48.3.2.2.1 Autoinjectors: Product Details
- 48.3.3. Union Medico
- 48.3.3.1. Company Overview
- 48.3.3.2. Product Portfolio
- 48.3.3.2.1. Autoinjectors: Product Details
- 48.3.3.2.1.1. 45⸰ Autoinjectors
- 48.3.3.2.1.2. 90⸰ Autoinjectors
- 48.3.3.2.1.3. SuperGrip Autoinjectors
- 48.3.3.2.1.4. Exclusive Autoinjectors
- 48.3.3.2.1. Autoinjectors: Product Details
- 48.3.4. SHL Group
- 48.3.4.1. Company Overview
- 48.3.4.2. Product Portfolio
- 48.3.4.2.1. Autoinjectors: Product Details (Based on Compatible Device)
- 48.3.4.2.1.1. Prefilled Syringe Based Autoinjectors
- 48.3.4.2.1.2. Cartridge Based Autoinjectors
- 48.3.4.2.2. Autoinjectors: Product Details (Based on Method of Preparation)
- 48.3.4.2.2.1. Two-Step Disposable Autoinjectors: Product Details
- 48.3.4.2.1. Autoinjectors: Product Details (Based on Compatible Device)
- 48.3.5. Ypsomed
- 48.3.5.1. Company Overview
- 48.3.5.2. Financial Information
- 48.3.5.3. Product Portfolio
- 48.3.5.3.1. Autoinjectors: Product Details
- 48.3.5.3.1.1. YpsoMate Series
- 48.3.5.3.1.2. VarioJect
- 48.3.5.3.1.3. LyoTwist Series
- 48.3.5.3.1. Autoinjectors: Product Details
- 48.3.1. Owen Mumford
49. CONCLUDING REMARKS
50. EXECUTIVE INSIGHTS
- 50.1. Chapter Overview
- 50.2. Oval Medical Technologies
- 50.2.1. Company Snapshot
- 50.2.2. Interview Transcript: Matthew Young, Founder and Chief Technology Officer
- 50.3. Intas Pharmaceuticals
- 50.3.1. Company Snapshot
- 50.3.2. Interview Transcript: Kirti Maheshwari, Chief Technical Officer
- 50.4. West Pharmaceutical
- 50.4.1. Company Snapshot
- 50.4.2. Interview Transcript: Tibor Hlobik, Senior Director and Kevin Cancelliere, Former Director, Pharmaceutical Delivery Systems Marketing
- 50.5. Lonstroff
- 50.5.1. Company Snapshot
- 50.5.2. Interview Transcript: Marco Pederiva, Head of R&D and Product Strategy
- 50.6. IDEO
- 50.6.1. Company Snapshot
- 50.6.2. Interview Transcript: Jesse Fourt, Senior Portfolio Director
- 50.7. IDT Biologika
- 50.7.1. Company Snapshot
- 50.7.2. Interview Transcript: Gregor Kawaletz, Former Chief Commercial Officer
- 50.8. Small Medical Device Company
- 50.8.1. Interview Transcript: Anonymous, Chief Executive Officer
51. APPENDIX 1: TABULATED DATA
52. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 5.1 Drugs: Shifted from Vials to Prefilled Syringe Systems
- Table 5.2 Components of Prefilled Syringes
- Table 5.3 Classification of Prefilled Syringes
- Table 5.4 Glass Barrel Prefilled Syringe Recalls
- Table 5.5 Pharmaceutical Filling Lines: Steps Performed by Type of Packaging of Prefilled Syringe
- Table 6.1 Prefilled Syringes: List of Products
- Table 6.2 Prefilled Syringes: List of Manufacturers
- Table 6.3 Prefilled Flush Syringes: List of Manufacturers
- Table 8.1 Prefilled Syringe Manufacturers (North America): List of Companies Profiled
- Table 8.2 Becton, Dickinson and Company: Company Snapshot
- Table 8.3 Becton, Dickinson and Company: Prefilled Syringes Portfolio
- Table 8.4 Becton, Dickinson and Company: Recent Developments and Future Outlook
- Table 8.5 Medefil: Company Snapshot
- Table 8.6 Medefil: Prefilled Syringes Portfolio
- Table 8.7 Medefil: Recent Developments and Future Outlook
- Table 8.8 West Pharmaceutical Services: Company Snapshot
- Table 8.9 West Pharmaceutical Services: Prefilled Syringes Portfolio
- Table 8.10 West Pharmaceutical Services: Recent Developments and Future Outlook
- Table 8.11 Medline: Company Snapshot
- Table 8.12 Medline: Prefilled Syringes Portfolio
- Table 8.13 MedXL: Company Snapshot
- Table 8.14 MedXL: Prefilled Syringes Portfolio
- Table 8.15 Pfizer: Company Snapshot
- Table 8.16 Pfizer: Prefilled Syringes Portfolio
- Table 9.1 Prefilled Syringe Manufacturers (Europe): List of Companies Profiled
- Table 9.2 Gerresheimer: Company Snapshot
- Table 9.3 Gerresheimer: Prefilled Syringes Portfolio
- Table 9.4 Gerresheimer: Recent Developments and Future Outlook
- Table 9.5 SCHOTT: Company Snapshot
- Table 9.6 SCHOTT: Prefilled Syringes Portfolio
- Table 9.7 SCHOTT: Recent Developments and Future Outlook
- Table 9.8 Stevanato: Company Snapshot
- Table 9.9 Stevanato: Prefilled Syringes Portfolio
- Table 9.10 Stevanato: Recent Developments and Future Outlook
- Table 9.11 B. Braun: Company Snapshot
- Table 9.12 B. Braun: Prefilled Syringes Portfolio
- Table 9.13 Nipro Europe: Company Snapshot
- Table 9.14 Nipro Europe: Prefilled Syringes Portfolio
- Table 9.15 Vetter Pharma: Company Snapshot
- Table 9.16 Vetter Pharma: Prefilled Syringes Portfolio
- Table 10.1 Prefilled Syringe Manufacturers (Asia-Pacific): List of Companies Profiled
- Table 10.2 Shandong Zibo Minkang Pharmaceutical Packing: Company Snapshot
- Table 10.3 Shandong Zibo Minkang Pharmaceutical Packing: Prefilled Syringes Portfolio
- Table 10.4 Shin Yan Sheno Precision Industrial: Company Snapshot
- Table 10.5 Shin Yan Sheno Precision Industrial: Prefilled Syringes Portfolio
- Table 10.6 Taisei Kako: Company Snapshot
- Table 10.7 Taisei Kako: Prefilled Syringes Portfolio
- Table 10.8 Taisei Kako: Recent Developments and Future Outlook
- Table 10.9 J O. Pharma: Company Snapshot
- Table 10.10 J O. Pharma: Prefilled Syringes Portfolio
- Table 10.11 Shandong Pharmaceutical Glass: Company Snapshot
- Table 10.12 Shandong Pharmaceutical Glass: Prefilled Syringes Portfolio
- Table 10.13 Shandong Weigao Group Medical Polymer: Company Snapshot
- Table 10.14 Shandong Weigao Group Medical Polymer: Prefilled Syringes Portfolio
- Table 11.1 Safety System: List of Add-on Safety Device Manufacturers
- Table 11.2 Safety System: List of Integrated Safety Device Manufacturers
- Table 12.1 FDA Center for Drug and Device Approval
- Table 12.2 Regulatory Review Timelines for Combination Products in the US
- Table 12.3 Data Requirements and Characteristics of Province-wide HTA Processes in Canada
- Table 12.4 Regulatory Bodies in EU5 Countries
- Table 12.5 Device Classification: Japan
- Table 12.6 Medical Device Reimbursement Categories
- Table 12.7 Device Classification: India
- Table 12.8 Device Classification: South Korea
- Table 12.9 Saudi Arabia: Regulatory Review Timelines
- Table 12.10 Device Classification: Brazil
- Table 12.11 Reimbursement and Pricing Approval Process of Prefilled Syringes in Australia
- Table 13.1 Prefilled Syringe Combination Products: List of Approved Drugs, since 2013
- Table 13.2 Prefilled Syringe Combination Products: Information on Approval Year and Region of Product Approval, since 2013
- Table 13.3 Prefilled Syringe Combination Products: Information on Other Approved Primary Packaging Systems
- Table 13.4 Prefilled Syringe Combination Products: Information on Dosage Strength
- Table 13.5 Prefilled Syringe Combination Products: List of Clinical Stage Drugs
- Table 13.6 Prefilled Syringe Combination Products: List of Developers
- Table 13.7 List of Other Drugs Available in Prefilled Syringes Format
- Table 14.1 Autoimmune Disorders: List of Leading Approved Drugs
- Table 14.2 Autoimmune Disorders: Information on Patent Expiry and Biosimilar Developed for Drugs in Prefilled Syringe Format
- Table 14.3 Infectious Diseases: List of Leading Approved Drugs
- Table 14.4 Neurological Disorders: List of Leading Approved Drugs
- Table 15.1 Most Likely Candidates for Delivery via Prefilled Syringes
- Table 15.2 Likely Candidates for Delivery via Prefilled Syringes
- Table 15.3 Less Likely Candidates for Delivery via Prefilled Syringes
- Table 17.1 Prefilled Syringes: List of Recent Developments, since 2019
- Table 18.1 Patent Analysis: Top CPC Sections
- Table 18.2 Patent Analysis: Top CPC Symbols
- Table 18.3 Patent Analysis: Top CPC Codes
- Table 18.4 Patent Analysis: Summary of Benchmarking Analysis
- Table 18.5 Patent Analysis: Categorization based on Weighted Valuation Scores
- Table 18.6 Patent Portfolio: List of Leading Patents (by Highest Relative Valuation)
- Table 18.5 Patent Portfolio: List of Leading Patents (by Number of Citations)
- Table 19.1 Prefilled Syringes: List of Key Opinion leaders
- Table 20.1 List of Prefilled Flush Syringes Available in the Market
- Table 20.2 List of Lyophilized Drugs Available with Prefilled Diluent Syringe System
- Table 20.3 Contrast Agents Available in Prefilled Syringes
- Table 21.1 List of Marketed Dual-Chamber Prefilled Syringes
- Table 22.1 Fraction of Global Population above 60 years, for 2022, 2030 and 2050 (in Millions)
- Table 22.2 List of Approved Autoinjector, since 2016
- Table 22.3 Marketed Autoinjectors / Pen-Injectors with Prefilled Syringes
- Table 23.1 List of Marketed / Pipeline Needleless Injection Devices
- Table 23.2 List of Prefilled Syringe Recalls, since 2003
- Table 46.1 Prefilled Syringe Component Manufacturers: List of Prefilled Syringe Component Manufacturers
- Table 46.2 Prominent Prefilled Syringes Component Manufacturers: List of Companies Profiled
- Table 46.3 West Pharmaceutical Services: Company Snapshot
- Table 46.4 West Pharmaceutical Services: Product Portfolio
- Table 46.5 Datwyler: Company Snapshot
- Table 46.6 Datwyler: Product Portfolio
- Table 46.7 Datwyler: Recent Developments and Future Outlook
- Table 46.8 Aptar Pharma: Company Snapshot
- Table 46.9 Aptar Pharma: Product Portfolio
- Table 46.10 Aptar Pharma: Recent Developments and Future Outlook Future Outlook
- Table 46.11 Stevanato: Company Snapshot
- Table 46.12 Stevanato: Product Portfolio
- Table 47.1 Prefilled Syringes: List of Fill / Finish Service Providers
- Table 48.1 List of Autoinjectors (Using Prefilled Syringes)
- Table 50.1 Oval Medical Technologies: Company Snapshot
- Table 50.2 Intas Pharmaceuticals: Company Snapshot
- Table 50.3 West Pharmaceutical: Company Snapshot
- Table 50.4 Lonstroff: Company Snapshot
- Table 50.5 IDEO: Company Snapshot
- Table 50.6 IDT Biologika: Company Snapshot
- Table 51.1 Prefilled Syringes: Distribution by Type of Barrel Fabrication Material
- Table 51.2 Prefilled Syringes: Distribution by Number of Barrel Chambers
- Table 51.3 Prefilled Syringes: Distribution by Type of Needle System
- Table 51.4 Prefilled Syringes: Distribution by Volume of Syringe
- Table 51.5 Prefilled Syringe Manufacturers: Distribution by Year of Establishment
- Table 51.6 Prefilled Syringe Manufacturers: Distribution by Company Size
- Table 51.7 Prefilled Syringe Manufacturers: Distribution by Location of Headquarters
- Table 51.8 Prefilled Syringe Manufacturers: Distribution by Company Size and Location of Headquarters
- Table 51.9 Prefilled Syringe Manufacturer: Distribution by Location of Manufacturing Facilities
- Table 51.10 Most Active Players: Distribution by Number of Prefilled Syringes
- Table 51.11 Becton, Dickinson and Company: Annual Revenues, FY 2015 Onwards (USD Million)
- Table 51.12 Becton, Dickinson and Company: Distribution of Revenues by Business Divisions, FY 2022
- Table 51.13 West Pharmaceutical Services: Annual Revenues, FY 2015 Onwards (USD Million)
- Table 51.14 West Pharmaceutical Services: Distribution of Revenues by Business Divisions, FY 2022
- Table 51.15 Gerresheimer: Annual Revenues, FY 2015 Onwards (EUR Million)
- Table 51.16 Gerresheimer: Distribution of Revenues by Business Divisions, FY 2022
- Table 51.17 SCHOTT: Annual Revenues, FY 2015 Onwards (EUR Million)
- Table 51.18 SCHOTT: Distribution of Revenues by Business Divisions, FY 2022
- Table 51.19 Stevanato: Annual Revenues, FY 2020 Onwards (EUR Million)
- Table 51.20 Stevanato: Distribution of Revenues by Business Divisions, FY 2022
- Table 51.21 Approved Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule Stored
- Table 51.22 Approved Prefilled Syringe Combination Products: Distribution by Approval Year
- Table 51.23 Approved Prefilled Syringe Combination Products: Distribution by Route of Administration
- Table 51.24 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Table 51.25 Approved Prefilled Syringe Combination Products: Distribution by Region of Product Approval
- Table 51.26 Approved Prefilled Syringe Combination Products: Distribution by Other Approved Primary Packaging System
- Table 51.27 Clinical Stage Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule Stored
- Table 51.28 Clinical Stage Prefilled Syringe Combination Products: Distribution by Phase of Development
- Table 51.29 Clinical Stage Prefilled Syringe Combination Products: Distribution by Route of Administration
- Table 51.30 Clinical Stage Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Table 51.31 Prefilled Syringe Combination Product Developers: Distribution by Year of Establishment
- Table 51.32 Prefilled Syringe Combination Product Developers: Distribution by Company Size
- Table 51.33 Prefilled Syringe Combination Product Developers: Distribution by Location of Headquarters
- Table 51.34 Sales of Leading Drugs Available in Prefilled Syringe Format, 2022 (USD Billion)
- Table 51.35 Humira: Annual Sales, FY 2010 Onwards (USD Million)
- Table 51.36 Recent Developments: Cumulative Year-wise Trend, since 2019
- Table 51.37 Recent Developments: Distribution by Type of Initiative
- Table 51.38 Recent Developments: Distribution by Year and Type of Initiative, since 2019
- Table 51.39 Recent Developments: Distribution by Type of Expansion
- Table 51.40 Recent Developments: Distribution by Location of Expansion
- Table 51.41 Recent Developments: Distribution by Type of Partnership
- Table 51.42 Recent Developments: Distribution by Geography
- Table 51.43 Most Active Players: Distribution by Number of Recent Initiatives
- Table 51.44 Most Active Players: Distribution by Type of Initiative
- Table 51.45 Patent Analysis: Distribution by Type of Patent
- Table 51.46 Patent Analysis: Cumulative Distribution by Patent Publication Year, since 2019
- Table 51.47 Patent Analysis: Distribution by Type of Patent and Publication Year, since 2019
- Table 51.48 Patent Analysis: Distribution by Patent Application Year
- Table 51.49 Patent Analysis: Distribution by Geography
- Table 51.50 Patent Analysis: Cumulative Year-wise Distribution by Type of Applicant, since 2019
- Table 51.51 Leading Players: Distribution by Number of Patents
- Table 51.52 Leading Individual Assignees: Distribution by Number of Patents
- Table 51.53 Patent Analysis: Distribution by Patent Age
- Table 51.54 Prefilled Syringes KOL Analysis: Distribution by Type of KOL
- Table 51.55 Prefilled Syringes KOL Analysis: Distribution by Type of Sponsor Organization
- Table 51.56 Prefilled Syringes KOL Analysis: Distribution by Affiliated Organization
- Table 51.57 Prefilled Syringes KOL Analysis: Distribution by Target Disease Indication
- Table 51.58 Prefilled Syringes KOL Analysis: Distribution by Geographical Location of KOL
- Table 51.59 Most Prominent KOLs: Distribution by RA Score
- Table 51.60 Global Prefilled Syringes Market, Historical Trends (since 2018) (USD Million)
- Table 51.61 Global Prefilled Syringes Market, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.62 Prefilled Syringes Market: Distribution by Purpose of Prefilled Syringe, 2018, 2023 and 2035 (USD Million)
- Table 51.63 Specialty Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.64 Specialty Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.65 Therapeutic Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.66 Therapeutic Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.67 Cosmetic Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.68 Cosmetic Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.69 Prefilled Syringes Market: Distribution by Type of Molecule, 2018, 2023 and 2035
- Table 51.70 Prefilled Syringes Market for Proteins, Historical Trends (since 2018) (USD Million)
- Table 51.71 Prefilled Syringes Market for Proteins, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.72 Prefilled Syringes Market for Antibodies, Historical Trends (since 2018) (USD Million)
- Table 51.73 Prefilled Syringes Market for Antibodies, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.74 Prefilled Syringes Market for Peptides, Historical Trends (since 2018) (USD Million)
- Table 51.75 Prefilled Syringes Market for Peptides, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.76 Prefilled Syringes Market for Small Molecules, Historical Trends (since 2018) (USD Million)
- Table 51.77 Prefilled Syringes Market for Small Molecules, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.78 Prefilled Syringes Market for Vaccines, Historical Trends (since 2018) (USD Million)
- Table 51.79 Prefilled Syringes Market for Vaccines, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.80 Prefilled Syringes Market for Cell Therapies, Historical Trends (since 2018) (USD Million)
- Table 51.81 Prefilled Syringes Market for Cell Therapies, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.82 Prefilled Syringes Market: Distribution by Therapeutic Area, 2018, 2023 and 2035
- Table 51.83 Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.84 Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.85 Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.86 Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.87 Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.88 Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.89 Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.90 Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.91 Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.92 Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.93 Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.94 Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.95 Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.96 Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.97 Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.98 Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.99 Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.100 Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.101 Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.102 Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.103 Prefilled Syringes Market for Other Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.104 Prefilled Syringes Market for Other Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.105 Prefilled Syringes Market: Distribution by Type of Barrel Material, 2018, 2023 and 2035 (USD Million)
- Table 51.106 Glass Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.107 Glass Prefilled Syringes, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.108 Plastic Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.109 Plastic Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.110 Prefilled Syringes Market: Distribution by Number of Barrel Chamber, 2018, 2023 and 2035
- Table 51.111 Single Chamber Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.112 Single Chamber Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.113 Dual Chamber Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.114 Dual Chamber Prefilled Syringes Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.115 Prefilled Syringes Market: Distribution by Type of Needle System, 2018, 2023 and 2035
- Table 51.116 Staked Needle Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.117 Staked Needle Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.118 Luer Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.119 Luer Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.120 Prefilled Syringes Market: Distribution by Usability of Syringe, 2018, 2023 and 2035
- Table 51.121 Disposable Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.122 Disposable Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.123 Reusable Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.124 Reusable Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.125 Prefilled Syringes Market: Distribution by Type of Syringe, 2018, 2023 and 2035
- Table 51.126 Conventional Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.127 Conventional Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.128 Safety Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.129 Safety Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.130 Prefilled Syringes Market: Distribution by Type of Packaging, 2018, 2023 and 2035
- Table 51.131 Bulk Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.132 Bulk Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.133 Nested Prefilled Syringes, Historical Trends (since 2018) (USD Million)
- Table 51.134 Nested Prefilled Syringes, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.135 Prefilled Syringes Market: Distribution by Key Geographical Regions, 2018, 2023 and 2035
- Table 51.136 Prefilled Syringes Market for North America, Historical Trends (since 2018) (USD Million)
- Table 51.137 Prefilled Syringes Market for North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.138 Prefilled Syringes Market for Europe, Historical Trends (since 2018) (USD Million)
- Table 51.139 Prefilled Syringes Market for Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.140 Prefilled Syringes Market for Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.141 Prefilled Syringes Market for Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.142 Prefilled Syringes Market for Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.143 Prefilled Syringes Market for Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.144 Prefilled Syringes Market for Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.145 Prefilled Syringes Market for Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.146 Proteins Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.147 Proteins Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.148 Small Molecules Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.149 Small Molecules Market for Blood Disorders, Forecasted Estimates (till 2035) (USD Million)
- Table 51.150 Glass Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.151 Glass Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.152 Plastic Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.153 Plastic Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.154 Single Chamber Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.155 Single Chamber Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.156 Dual Chamber Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.157 Dual Chamber Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.158 Staked Needle Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.159 Staked Needle Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.160 Luer Needle Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.161 Luer Needle Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.162 Disposable Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.163 Disposable Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.164 Reusable Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.165 Reusable Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.166 Conventional Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.167 Conventional Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.168 Safety Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.169 Safety Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.170 Bulk Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.171 Bulk Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.172 Nested Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.173 Nested Prefilled Syringes Market for Blood Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.174 Prefilled Syringes Market for Blood Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.175 Prefilled Syringes Market for Blood Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.176 Prefilled Syringes Market for Blood Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.177 Prefilled Syringes Market for Blood Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.178 Prefilled Syringes Market for Blood Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.179 Prefilled Syringes Market for Blood Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.180 Prefilled Syringes Market for Blood Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.181 Prefilled Syringes Market for Blood Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.182 Prefilled Syringes Market for Blood Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.183 Prefilled Syringes Market for Blood Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.184 Proteins Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.185 Proteins Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.186 Vaccines Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.187 Vaccines Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.188 Glass Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.189 Glass Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.190 Plastic Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.191 Plastic Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.192 Single Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.193 Single Chamber Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.194 Dual Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.195 Dual Chamber Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.196 Staked Needle Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.197 Staked Needle Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.198 Luer Prefilled Syringes Market for Infectious Disease, Historical Trends (since 2018) (USD Million)
- Table 51.199 Luer Prefilled Syringes Market for Infectious Disease, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.200 Disposable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.201 Disposable Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.202 Reusable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.203 Reusable Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.204 Conventional Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.205 Conventional Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.206 Safety Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.207 Safety Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.208 Bulk Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.209 Bulk Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.210 Nested Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) (USD Million)
- Table 51.211 Nested Prefilled Syringes Market for Infectious Diseases, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.212 Prefilled Syringes Market for Infectious Diseases in North America, Historical Trends (since 2018) (USD Million)
- Table 51.213 Prefilled Syringes Market for Infectious Diseases in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.214 Prefilled Syringes Market for Infectious Diseases in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.215 Prefilled Syringes Market for Infectious Diseases in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.216 Prefilled Syringes Market for Infectious Diseases in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.217 Prefilled Syringes Market for Infectious Diseases in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.218 Prefilled Syringes Market for Infectious Diseases in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.219 Prefilled Syringes Market for Infectious Diseases in Middle East and North Africa, Forecasted Estimates (till 2035)
- Table 51.220 Prefilled Syringes Market for Infectious Diseases in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.221 Prefilled Syringes Market for Infectious Diseases in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.222 Proteins Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.223 Proteins Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.224 Antibodies Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.225 Antibodies Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.226 Peptides Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.227 Peptides Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.228 Small Molecule Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.229 Small Molecule Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.230 Glass Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.231 Glass Barrel Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.232 Plastic Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.233 Plastic Barrel Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.234 Single Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.235 Single Chamber Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.236 Dual Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.237 Dual Chamber Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.238 Staked Needle Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.239 Staked Needle Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.240 Luer Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.241 Luer Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.242 Disposable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.243 Disposable Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.244 Reusable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.245 Reusable Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.246 Conventional Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.247 Conventional Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.248 Safety Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.249 Safety Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.250 Bulk Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.251 Bulk Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.252 Nested Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.253 Nested Prefilled Syringes Market for Autoimmune Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.254 Prefilled Syringes Market for Autoimmune Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.255 Prefilled Syringes Market for Autoimmune Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.256 Prefilled Syringes Market for Autoimmune Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.257 Prefilled Syringes Market for Autoimmune Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.258 Prefilled Syringes Market for Autoimmune Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.259 Prefilled Syringes Market for Autoimmune Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.260 Prefilled Syringes Market for Autoimmune Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.261 Prefilled Syringes Market for Autoimmune Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.262 Prefilled Syringes Market for Autoimmune Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.263 Prefilled Syringes Market for Autoimmune Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.264 Proteins Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.265 Proteins Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.266 Antibodies Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.267 Antibodies Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.268 Peptides Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.269 Peptides Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.270 Small Molecules Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.271 Small Molecules Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.272 Vaccines Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.273 Vaccines Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.274 Glass Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.275 Glass Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.276 Plastic Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.277 Plastic Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.278 Single Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.279 Single Chamber Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.280 Dual Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.281 Dual Chamber Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.282 Staked Needle Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.283 Staked Needle Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.284 Luer Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.285 Luer Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.286 Disposable Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.287 Disposable Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.288 Reusable Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.289 Reusable Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.290 Conventional Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.291 Conventional Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.292 Safety Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.293 Safety Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.294 Bulk Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.295 Bulk Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.296 Nested Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.297 Nested Prefilled Syringes Market for Oncological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.298 Prefilled Syringes Market for Oncological Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.299 Prefilled Syringes Market for Oncological Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.300 Prefilled Syringes Market for Oncological Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.301 Prefilled Syringes Market for Oncological Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.302 Prefilled Syringes Market for Oncological Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.303 Prefilled Syringes Market for Oncological Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.304 Prefilled Syringes Market for Oncological Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.305 Prefilled Syringes Market for Oncological Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.306 Prefilled Syringes Market for Oncological Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.307 Prefilled Syringes Market for Oncological Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.308 Antibodies Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.309 Antibodies Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.310 Cell Therapies Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.311 Cell Therapies Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.312 Glass Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.313 Glass Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.314 Plastic Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.315 Plastic Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.316 Single Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.317 Single Chamber Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.318 Dual Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.319 Dual Chamber Prefilled Syringes Market for Cardiovascular Disorders Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.320 Staked Needle Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.321 Staked Needle Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.322 Luer Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.323 Luer Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.324 Disposable Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.325 Disposable Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.326 Reusable Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.327 Reusable Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.328 Conventional Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.329 Conventional Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.330 Safety Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.331 Safety Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.332 Bulk Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.333 Bulk Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.334 Nested Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.335 Nested Prefilled Syringes Market for Cardiovascular Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.336 Prefilled Syringes Market for Cardiovascular Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.337 Prefilled Syringes Market for Cardiovascular Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.338 Prefilled Syringes Market for Cardiovascular Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.339 Prefilled Syringes Market for Cardiovascular Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.340 Prefilled Syringes Market for Cardiovascular Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.341 Prefilled Syringes Market for Cardiovascular Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.342 Prefilled Syringes Market for Cardiovascular Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.343 Prefilled Syringes Market for Cardiovascular Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.344 Prefilled Syringes Market for Cardiovascular Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.345 Prefilled Syringes Market for Cardiovascular Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.346 Antibodies Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.347 Antibodies Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.348 Glass Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.349 Glass Barrel Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.350 Plastic Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.351 Plastic Barrel Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.352 Single Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.353 Single Chamber Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.354 Dual Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.355 Dual Chamber Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.356 Staked Needle Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.357 Staked Needle Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.358 Luer Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.359 Luer Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.360 Disposable Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.361 Disposable Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.362 Reusable Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.363 Reusable Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.364 Conventional Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.365 Conventional Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.366 Safety Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.367 Safety Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.368 Bulk Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.369 Bulk Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.370 Nested Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.371 Nested Prefilled Syringes Market for Respiratory Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.372 Prefilled Syringes Market for Respiratory Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.373 Prefilled Syringes Market for Respiratory Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.374 Prefilled Syringes Market for Respiratory Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.375 Prefilled Syringes Market for Respiratory Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.376 Prefilled Syringes Market for Respiratory Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.377 Prefilled Syringes Market for Respiratory Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.378 Prefilled Syringes Market for Respiratory Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.379 Prefilled Syringes Market for Respiratory Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.380 Prefilled Syringes Market for Respiratory Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.381 Prefilled Syringes Market for Respiratory Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.382 Antibodies Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.383 Antibodies Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.384 Proteins Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.385 Proteins Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.386 Peptides Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.387 Peptides Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.388 Small Molecules Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.389 Small Molecules Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.390 Glass Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.391 Glass Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.392 Plastic Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.393 Plastic Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.394 Single Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.395 Single Chamber Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.396 Dual Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.397 Dual Chamber Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.398 Staked Needle Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.399 Staked Needle Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.400 Luer Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.401 Luer Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.402 Disposable Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.403 Disposable Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.404 Reusable Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.405 Reusable Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.406 Conventional Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.407 Conventional Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.408 Safety Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.409 Safety Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.410 Bulk Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.411 Bulk Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.412 Nested Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.413 Nested Prefilled Syringes Market for Neurological Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.414 Prefilled Syringes Market for Neurological Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.415 Prefilled Syringes Market for Neurological Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.416 Prefilled Syringes Market for Neurological Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.417 Prefilled Syringes Market for Neurological Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.418 Prefilled Syringes Market for Neurological Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.419 Prefilled Syringes Market for Neurological Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.420 Prefilled Syringes Market for Neurological Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.421 Prefilled Syringes Market for Neurological Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.422 Prefilled Syringes Market for Neurological Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.423 Prefilled Syringes Market for Neurological Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.424 Proteins Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.425 Proteins Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.426 Small Molecule Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.427 Small Molecule Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.428 Peptides Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.429 Peptides Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.430 Glass Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.431 Glass Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.432 Plastic Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.433 Plastic Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.434 Single Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.435 Single Chamber Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.436 Dual Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.437 Dual Chamber Prefilled Syringes Market for Metabolic Disorders Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.438 Staked Needle Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.439 Staked Needle Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.440 Luer Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.441 Luer Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.442 Disposable Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.443 Disposable Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.444 Reusable Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.445 Reusable Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.446 Conventional Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.447 Conventional Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.448 Safety Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.449 Safety Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.450 Bulk Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.451 Bulk Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.452 Nested Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.453 Nested Prefilled Syringes Market for Metabolic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.454 Prefilled Syringes Market for Metabolic Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.455 Prefilled Syringes Market for Metabolic Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.456 Prefilled Syringes Market for Metabolic Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.457 Prefilled Syringes Market for Metabolic Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.458 Prefilled Syringes Market for Metabolic Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.459 Prefilled Syringes Market for Metabolic Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.460 Prefilled Syringes Market for Metabolic Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.461 Prefilled Syringes Market for Metabolic Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.462 Prefilled Syringes Market for Metabolic Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.463 Prefilled Syringes Market for Metabolic Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.464 Proteins Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.465 Proteins Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.466 Antibodies Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.467 Antibodies Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.468 Glass Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.469 Glass Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.470 Plastic Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.471 Plastic Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.472 Single Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.473 Single Chamber Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.474 Dual Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.475 Dual Chamber Prefilled Syringes Market for Ophthalmic Disorders Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.476 Staked Needle Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.477 Staked Needle Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.478 Luer Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.479 Luer Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.480 Disposable Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.481 Disposable Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.482 Reusable Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.483 Reusable Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.484 Conventional Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.485 Conventional Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.486 Safety Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.487 Safety Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.488 Bulk Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.489 Bulk Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.490 Nested Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.491 Nested Prefilled Syringes Market for Ophthalmic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.492 Prefilled Syringes Market for Ophthalmic Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.493 Prefilled Syringes Market for Ophthalmic Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.494 Prefilled Syringes Market for Ophthalmic Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.495 Prefilled Syringes Market for Ophthalmic Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.496 Prefilled Syringes Market for Ophthalmic Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.497 Prefilled Syringes Market for Ophthalmic Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.498 Prefilled Syringes Market for Ophthalmic Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.499 Prefilled Syringes Market for Ophthalmic Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.500 Prefilled Syringes Market for Ophthalmic Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.501 Prefilled Syringes Market for Ophthalmic Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.502 Antibodies Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.503 Antibodies Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.504 Small Molecules Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.505 Small Molecules Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.506 Cell Therapies Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.507 Cell Therapies Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.508 Glass Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.509 Glass Barrel Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.510 Plastic Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.511 Plastic Barrel Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.512 Single Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.513 Single Chamber Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.514 Dual Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.515 Dual Chamber Prefilled Syringes Market for Orthopedic Disorders Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.516 Staked Needle Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.517 Staked Needle Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.518 Luer Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.519 Luer Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.520 Disposable Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.521 Disposable Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.522 Reusable Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.523 Reusable Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.524 Conventional Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.525 Conventional Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.526 Safety Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.527 Safety Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.528 Bulk Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.529 Bulk Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.530 Nested Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.531 Nested Prefilled Syringes Market for Orthopedic Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.532 Prefilled Syringes Market for Orthopedic Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.533 Prefilled Syringes Market for Orthopedic Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.534 Prefilled Syringes Market for Orthopedic Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.535 Prefilled Syringes Market for Orthopedic Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.536 Prefilled Syringes Market for Orthopedic Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.537 Prefilled Syringes Market for Orthopedic Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.538 Prefilled Syringes Market for Orthopedic Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.539 Prefilled Syringes Market for Orthopedic Disorders in Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.540 Prefilled Syringes Market for Orthopedic Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.541 Prefilled Syringes Market for Orthopedic Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.542 Proteins Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.543 Proteins Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.544 Antibodies Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.545 Antibodies Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.546 Small Molecules Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.547 Small Molecules Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.548 Peptides Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.549 Peptides Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.550 Vaccines Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.551 Vaccines Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.552 Cell Therapies Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.553 Cell Therapies Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.554 Glass Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.555 Glass Barrel Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035) Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.556 Plastic Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.557 Plastic Barrel Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.558 Single Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.559 Single Chamber Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.560 Dual Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.561 Dual Chamber Prefilled Syringes Market for Other Disorders Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.562 Staked Needle Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.563 Staked Needle Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.564 Luer Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.565 Luer Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.566 Disposable Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.567 Disposable Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.568 Reusable Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.569 Reusable Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.570 Conventional Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.571 Conventional Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.572 Safety Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.573 Safety Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.574 Bulk Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.575 Bulk Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.576 Nested Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) (USD Million)
- Table 51.577 Nested Prefilled Syringes Market for Other Disorders, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.578 Prefilled Syringes Market for Other Disorders in North America, Historical Trends (since 2018) (USD Million)
- Table 51.579 Prefilled Syringes Market for Other Disorders in North America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.580 Prefilled Syringes Market for Other Disorders in Europe, Historical Trends (since 2018) (USD Million)
- Table 51.581 Prefilled Syringes Market for Other Disorders in Europe, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.582 Prefilled Syringes Market for Other Disorders in Asia-Pacific, Historical Trends (since 2018) (USD Million)
- Table 51.583 Prefilled Syringes Market for Other Disorders in Asia-Pacific, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.584 Prefilled Syringes Market for Other Disorders in Middle East and North Africa, Historical Trends (since 2018) (USD Million)
- Table 51.585 Prefilled Syringes Market for Other Disorders: Middle East and North Africa, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.586 Prefilled Syringes Market for Other Disorders in Latin America, Historical Trends (since 2018) (USD Million)
- Table 51.587 Prefilled Syringes Market for Other Disorders in Latin America, Forecasted Estimates (till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 51.588 Datwyler: Annual Revenues, FY 2015 Onwards (CHF Billion)
- Table 51.589 Aptar Pharma: Annual Revenues, FY 2015 Onwards (USD Billion)
- Table 51.590 Prefilled Syringes Fill / Finish Service Providers: Distribution by Year of Establishment
- Table 51.591 Prefilled Syringe Fill / Finish Service Providers: Distribution by Scale of Operation (Under Development / Approved)
List of Figures
- Figure 2.1 Research Methodology: Project Methodology
- Figure 2.2 Research Methodology: Forecast Methodology
- Figure 2.3 Research Methodology: Robust Quality Control
- Figure 2.4 Research Methodology: Key Market Segmentations
- Figure 3.1 Lessons Learnt from Past Recessions
- Figure 4.1 Executive Summary: Prefilled Syringes Market Landscape (I/II)
- Figure 4.2 Executive Summary: Prefilled Syringes Market Landscape (I/II)
- Figure 4.3 Executive Summary: Prefilled Syringe Combination Products Market Landscape
- Figure 4.4 Executive Summary: Recent Developments
- Figure 4.5 Executive Summary: Patent Analysis
- Figure 4.6 Executive Summary: Market Forecast and Opportunity Analysis (I/II)
- Figure 4.7 Executive Summary: Market Forecast and Opportunity Analysis (II/II)
- Figure 5.1 Glass Barrel Prefilled Syringes: Benefits and Limitations
- Figure 5.2 Critical Attributes of a Prefilled Syringe Design
- Figure 5.3 Key Steps Involved in Prefilled Syringe Manufacturing
- Figure 6.1 Prefilled Syringes: Distribution by Type of Barrel Fabrication Material
- Figure 6.2 Prefilled Syringes: Distribution by Number of Barrel Chambers
- Figure 6.3 Prefilled Syringes: Distribution by Type of Needle System
- Figure 6.4 Prefilled Syringes: Distribution by Volume of Syringe
- Figure 6.5 Prefilled Syringe Manufacturers: Distribution by Year of Establishment
- Figure 6.6 Prefilled Syringe Manufacturers: Distribution by Company Size
- Figure 6.7 Prefilled Syringe Manufacturers: Distribution by Location of Headquarters
- Figure 6.8 Prefilled Syringe Manufacturers: Distribution by Company Size and Location of Headquarters
- Figure 6.9 Prefilled Syringe Manufacturers: Distribution by Location of Manufacturing Facilities
- Figure 6.10 Most Active Players: Distribution by Number of Prefilled Syringes
- Figure 7.1 Product Competitiveness Analysis: Glass Barrel Prefilled Syringes
- Figure 7.2 Product Competitiveness Analysis: Plastic Barrel Prefilled Syringes
- Figure 8.1 Becton, Dickinson and Company: Annual Revenues, FY 2015 Onwards (USD Million)
- Figure 8.2 Becton, Dickinson and Company: Distribution of Revenues by Business Divisions, FY 2022
- Figure 8.3 West Pharmaceutical Services: Annual Revenues, FY 2015 Onwards (USD Million)
- Figure 8.4 West Pharmaceutical Services: Distribution of Revenues by Business Divisions, FY 2022
- Figure 9.1 Gerresheimer: Annual Revenues, FY 2015 Onwards (EUR Million)
- Figure 9.2 Gerresheimer: Distribution of Revenues by Business Divisions, FY 2022
- Figure 9.3 SCHOTT: Annual Revenues, FY 2015 Onwards (EUR Million)
- Figure 9.4 SCHOTT: Distribution of Revenues by Business Divisions, FY 2022
- Figure 9.5 Stevanato: Annual Revenues, FY 2020 Onwards (EUR Million)
- Figure 9.6 Stevanato: Distribution of Revenues by Business Divisions, FY 2022
- Figure 11.1 Evolution of Global Healthcare Safety Legislations Across the Globe
- Figure 12.1 Global Regulations Related to Prefilled Syringes
- Figure 12.2 Approval Pathway for Combination Products in the US
- Figure 12.3 Healthcare Insurance Coverage in the US: Distribution by Type of Coverage
- Figure 12.4 Prefilled Syringes National Coverage Determination Process in the US
- Figure 12.5 Approval Pathway for Combination Products in Canada
- Figure 12.6 Healthcare Insurance Coverage in Canada: Distribution by Type of Coverage
- Figure 12.7 Healthcare Insurance Coverage in the UK: Distribution by Type of Coverage
- Figure 12.8 Reimbursement Process for Prefilled Syringes in the UK
- Figure 12.9 Healthcare Insurance Coverage in France: Distribution by Type of Coverage
- Figure 12.10 Reimbursement Process for Prefilled Syringes through LPPR
- Figure 12.11 Reimbursement Process for Prefilled Syringes in Spain
- Figure 12.12 Reimbursement Process for In-Patient Setting in Germany
- Figure 12.13 Reimbursement Process for Out-Patient Care in Germany
- Figure 12.14 Reimbursement Process for Prefilled Syringes in Italy
- Figure 12.15 ' Pharmaceuticals and Prefilled Syringes Agency: Review / Approval Process
- Figure 12.16 Snapshot of the Regulatory and Reimbursement Process in Japan
- Figure 12.17 Healthcare Insurance Coverage in Japan: Distribution by Type of Coverage
- Figure 12.18 Reimbursement Process for New Prefilled Syringes in Japan
- Figure 12.19 Approval Pathway for Combination Products in China
- Figure 12.20 Healthcare Insurance Coverage in China: Distribution by Type of Coverage
- Figure 12.21 Reimbursement Approval Process for Prefilled Syringes in Shanghai
- Figure 12.22 Healthcare Insurance Coverage in South Korea: Distribution by Type of Coverage
- Figure 12.23 Healthcare Insurance Coverage in Saudi Arabia: Distribution by Type of Coverage
- Figure 12.24 Reimbursement Process for Prefilled Syringes in Brazil
- Figure 12.25 Healthcare Insurance Coverage in Australia: Distribution by Type of Coverage
- Figure 13.1 Approved Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule Stored
- Figure 13.2 Approved Prefilled Syringe Combination Products: Distribution by Approval Year
- Figure 13.3 Approved Prefilled Syringe Combination Products: Distribution by Route of Administration
- Figure 13.4 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Figure 13.5 Approved Prefilled Syringe Combination Products: Distribution by Region of Product Approval
- Figure 13.6 Approved Prefilled Syringe Combination Products: Distribution by Other Approved Primary Packaging Systems
- Figure 13.7 Clinical Stage Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule Stored
- Figure 13.8 Clinical Stage Prefilled Syringe Combination Products: Distribution by Phase of Development
- Figure 13.9 Clinical Stage Prefilled Syringe Combination Products: Distribution by Route of Administration
- Figure 13.10 Clinical Stage Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Figure 13.11 Prefilled Syringe Combination Product Developers: Distribution by Year of Establishment
- Figure 13.12 Prefilled Syringe Combination Product Developers: Distribution by Company Size
- Figure 13.13 Prefilled Syringe Combination Product Developers: Distribution by Location of Headquarters
- Figure 13.14 Sales of Leading Drugs Available in Prefilled Syringe Format, FY 2022 (USD Billion)
- Figure 13.15 HUMIRA: Annual Sales, FY 2010 Onwards (USD Million)
- Figure 16.1 Big Pharma Players Combination Product Portfolio: Distribution by Target Therapeutic Areas
- Figure 16.2 Big Pharma Players Combination Product Portfolio for Autoimmune Disorders: Distribution by Stage of Development
- Figure 16.3 Big Pharma Players Combination Product Portfolio for Autoimmune Disorders: Distribution by Target Indication
- Figure 16.4 Big Pharma Players Combination Product Portfolio for Infectious Diseases: Distribution by Stage of Development
- Figure 16.5 Big Pharma Players Combination Product Portfolio for Infectious Diseases: Distribution by Target Indication
- Figure 16.6 Big Pharma Players Combination Product Portfolio for Neurological Disorders: Distribution by Stage of Development
- Figure 16.7 Big Pharma Players Combination Product Portfolio for Neurological Disorders: Distribution by Target Indication
- Figure 16.8 Big Pharma Players Combination Product Portfolio for Blood Disorders: Distribution by Stage of Development
- Figure 16.9 Big Pharma Players Combination Product Portfolio for Blood Disorders: Distribution by Target Indication
- Figure 16.10 Big Pharma Players Combination Product Portfolio for Oncological Disorders: Distribution by Stage of Development
- Figure 16.11 Big Pharma Players Combination Product Portfolio for Oncological Disorders: Distribution by Target Indication
- Figure 16.12 Big Pharma Players Combination Product Portfolio: Distribution by Type of Molecule
- Figure 16.13 Big Pharma Players Combination Product Portfolio for Antibodies: Distribution by Stage of Development
- Figure 16.14 Big Pharma Players Combination Product Portfolio Vaccines: Distribution by Stage of Development
- Figure 16.15 Big Pharma Players Combination Product Portfolio Proteins: Distribution by Stage of Development
- Figure 16.16 Big Pharma Players Combination Product Portfolio Peptides: Distribution by Stage of Development
- Figure 16.17 Big Pharma Players Combination Product Portfolio Small Molecules: Distribution by Stage of Development
- Figure 16.18 Big Pharma Players Combination Product Portfolio Other Biologics: Distribution by Stage of Development
- Figure 17.1 Recent Developments: Cumulative Year-wise Trend, since 2019
- Figure 17.2 Recent Developments: Distribution by Type of Initiative
- Figure 17.3 Recent Developments: Distribution by Year and Type of Initiative
- Figure 17.4 Recent Developments: Distribution by Type of Expansion
- Figure 17.5 Recent Developments: Distribution by Location of Expansion
- Figure 17.6 Recent Developments: Distribution by Type of Partnership
- Figure 17.7 Recent Developments: Distribution by Geography
- Figure 17.8 Most Active Players: Distribution by Number of Recent Initiatives
- Figure 17.9 Most Active Players: Distribution by Type of Initiatives
- Figure 18.1 Patent Analysis: Distribution by Type of Patent
- Figure 18.2 Patent Analysis: Cumulative Distribution by Patent Publication Year, since 2019
- Figure 18.3 Patent Analysis: Distribution by Type of Patent and Publication Year
- Figure 18.4 Patent Analysis: Distribution by Patent Application Year
- Figure 18.5 Patent Analysis: Distribution by Patent Jurisdiction
- Figure 18.6 Patent Analysis: Distribution by CPC Symbols
- Figure 18.7 Patent Analysis: Cumulative Year-wise Distribution by Type of Applicant, since 2019
- Figure 18.8 Leading Players: Distribution by Number of Patents
- Figure 18.9 Leading Individual Assignees: Distribution by Number of Patents
- Figure 18.10 Patent Benchmarking Analysis: Distribution of Leading Players by Patent Characteristics (CPC Codes)
- Figure 18.11 Patent Analysis: Distribution by Patent Age
- Figure 18.12 Prefilled Syringes: Patent Valuation
- Figure 19.1 Prefilled Syringes KOL Analysis: Distribution by Role of KOL
- Figure 19.2 Prefilled Syringes KOL Analysis: Distribution by Type of Sponsor Organization
- Figure 19.3 Prefilled Syringes KOL Analysis: Distribution by Affiliated Organization
- Figure 19.4 Prefilled Syringes KOL Analysis: Distribution by Target Disease Indication
- Figure 19.5 Prefilled Syringes KOL Analysis: Distribution by Geographical Location
- Figure 19.6 Prefilled Syringes Most Prominent KOLs: Distribution by Activeness, Expertise and Strength of KOL
- Figure 19.7 Prefilled Syringes Most Prominent KOLs: Distribution by RA Score
- Figure 22.1 Diabetes: Geographical Prevalence and Mortality, 2021 (in Millions)
- Figure 23.1 Prefilled Syringes: SWOT Analysis
- Figure 23.2 Prefilled Syringe Recalls: Distribution by Reason of Recall, since 2003
- Figure 23.3 Comparison of SWOT Factors: Harvey Ball Analysis
- Figure 24.1 Global Prefilled Syringes Market, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 24.2 Global Prefilled Syringes Market, Forecasted Estimates (till 2035): Conservative Scenario (USD Million)
- Figure 24.3 Global Prefilled Syringes Market, Forecasted Estimates (till 2035): Optimistic Scenario (USD Million)
- Figure 25.1 Prefilled Syringes Market: Distribution by Purpose of Prefilled Syringe, 2018, 2023 and 2035
- Figure 25.2 Specialty Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 25.3 Therapeutic Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 25.4 Cosmetic Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.1 Prefilled Syringes Market: Distribution by Type of Molecule, 2018, 2023 and 2035
- Figure 26.2 Prefilled Syringes Market for Proteins: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.3 Prefilled Syringes Market for Antibodies: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.4 Prefilled Syringes Market for Peptides: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.5 Prefilled Syringes Market for Small Molecules: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.6 Prefilled Syringes Market for Vaccines: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 26.7 Prefilled Syringes Market for Cell Therapies: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.1 Prefilled Syringes Market: Distribution by Target Therapeutic Area, 2018, 2023 and 2035
- Figure 27.2 Prefilled Syringes Market for Blood Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.3 Prefilled Syringes Market for Infectious Diseases: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.4 Prefilled Syringes Market for Autoimmune Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.5 Prefilled Syringes Market for Oncological Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.6 Prefilled Syringes Market for Cardiovascular Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.7 Prefilled Syringes Market for Respiratory Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.8 Prefilled Syringes Market for Neurological Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.9 Prefilled Syringes Market for Metabolic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.10 Prefilled Syringes Market for Ophthalmic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.11 Prefilled Syringes Market for Orthopedic Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 27.12 Prefilled Syringes Market for Other Disorders: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 28.1 Prefilled Syringes Market: Distribution by Type of Barrel Material, 2018, 2023 and 2035
- Figure 28.2 Glass Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 28.3 Plastic Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 29.1 Prefilled Syringes Market: Distribution by Number of Barrel Chamber, 2018, 2023 and 2035
- Figure 29.2 Single Chamber Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 29.3 Dual Chamber Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 30.1 Prefilled Syringes Market: Distribution by Type of Needle System, 2018, 2023 and 2035
- Figure 30.2 Staked Needle Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 30.3 Luer Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 31.1 Prefilled Syringes Market: Distribution by Usability of Syringe, 2018, 2023 and 2035
- Figure 31.2 Disposable Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 31.3 Reusable Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 32.1 Prefilled Syringes Market: Distribution by Type of Syringe, 2018, 2023 and 2035
- Figure 32.2 Conventional Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 32.3 Safety Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 33.1 Prefilled Syringes Market: Distribution by Type of Packaging, 2018, 2023 and 2035
- Figure 33.2 Bulk Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 33.3 Nested Prefilled Syringes: Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 34.1 Prefilled Syringes Market: Distribution by Key Geographical Regions, 2018, 2023 and 2035
- Figure 34.2 Prefilled Syringes Market in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 34.3 Prefilled Syringes Market in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 34.4 Prefilled Syringes Market in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 34.5 Prefilled Syringes Market in Middle East and North Africa Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 34.6 Prefilled Syringes Market in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.1 Proteins Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.2 Small Molecules Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.3 Glass Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.4 Plastic Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.5 Single Chamber Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.6 Dual Chamber Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.7 Staked Needle Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.8 Luer Needle Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.9 Disposable Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.10 Reusable Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.11 Conventional Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.12 Safety Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.13 Bulk Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.14 Nested Prefilled Syringes Market for Blood Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.15 Prefilled Syringes Market for Blood Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.16 Prefilled Syringes Market for Blood Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.17 Prefilled Syringes Market for Blood Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.18 Prefilled Syringes Market for Blood Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 35.19 Prefilled Syringes Market for Blood Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.1 Proteins Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.2 Vaccines Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.3 Glass Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.4 Plastic Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.5 Single Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.6 Dual Chamber Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.7 Staked Needle Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.8 Luer Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.9 Disposable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.10 Reusable Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.11 Conventional Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.12 Safety Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.13 Bulk Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.14 Nested Prefilled Syringes Market for Infectious Diseases, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.15 Prefilled Syringes Market for Infectious Diseases in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.16 Prefilled Syringes Market for Infectious Diseases in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.17 Prefilled Syringes Market for Infectious Diseases in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.18 Prefilled Syringes Market for Infectious Diseases in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 36.19 Prefilled Syringes Market for Infectious Diseases in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.1 Proteins Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.2 Antibodies Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.3 Peptides Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.4 Small Molecule Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.5 Glass Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.6 Plastic Barrel Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.7 Single Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.8 Dual Chamber Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.9 Staked Needle Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.10 Luer Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.11 Disposable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.12 Reusable Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.13 Conventional Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.14 Safety Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.15 Bulk Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.16 Nested Prefilled Syringes Market for Autoimmune Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.17 Prefilled Syringes Market for Autoimmune Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.18 Prefilled Syringes Market for Autoimmune Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.19 Prefilled Syringes Market for Autoimmune Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.20 Prefilled Syringes Market for Autoimmune Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 37.21 Prefilled Syringes Market for Autoimmune Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.1 Proteins Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.2 Antibodies Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.3 Peptides Market for Oncological Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.4 Small Molecules Market for Oncological Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.5 Vaccines Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.6 Glass Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.7 Plastic Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.8 Single Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.9 Dual Chamber Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.10 Staked Needle Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.11 Luer Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.12 Disposable Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.13 Reusable Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.14 Conventional Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.15 Safety Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.16 Bulk Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.17 Nested Prefilled Syringes Market for Oncological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.18 Prefilled Syringes Market for Oncological Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.19 Prefilled Syringes Market for Oncological Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.20 Prefilled Syringes Market for Oncological Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.21 Prefilled Syringes Market for Oncological Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 38.22 Prefilled Syringes Market for Oncological Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.1 Antibodies Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.2 Cell Therapies Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.3 Glass Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.4 Plastic Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.5 Single Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.6 Dual Chamber Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.7 Staked Needle Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.8 Luer Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.9 Disposable Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.10 Reusable Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.11 Conventional Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.12 Safety Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.13 Bulk Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.14 Nested Prefilled Syringes Market for Cardiovascular Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.15 Prefilled Syringes Market for Cardiovascular Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.16 Prefilled Syringes Market for Cardiovascular Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.17 Prefilled Syringes Market for Cardiovascular Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.18 Prefilled Syringes Market for Cardiovascular Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 39.19 Prefilled Syringes Market for Cardiovascular Disorders: Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.1 Antibodies Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.2 Glass Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.3 Plastic Barrel Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.4 Single Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.5 Dual Chamber Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.6 Staked Needle Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.7 Luer Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.8 Disposable Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.9 Reusable Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.10 Conventional Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.11 Safety Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.12 Bulk Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.13 Nested Prefilled Syringes Market for Respiratory Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.14 Prefilled Syringes Market for Respiratory Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.15 Prefilled Syringes Market for Respiratory Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.16 Prefilled Syringes Market for Respiratory Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.17 Prefilled Syringes Market for Respiratory Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 40.18 Prefilled Syringes Market for Respiratory Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.1 Antibodies Market for Neurological Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.2 Proteins Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.3 Peptides Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.4 Small Molecules Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.5 Glass Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.6 Plastic Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.7 Single Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.8 Dual Chamber Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.9 Staked Needle Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.10 Luer Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.11 Disposable Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.12 Reusable Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.13 Conventional Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.14 Safety Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.15 Bulk Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.16 Nested Prefilled Syringes Market for Neurological Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.17 Prefilled Syringes Market for Neurological Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.18 Prefilled Syringes Market for Neurological Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.19 Prefilled Syringes Market for Neurological Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.20 Prefilled Syringes Market for Neurological Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 41.21 Prefilled Syringes Market for Neurological Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.1 Proteins Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.2 Small Molecule Market for Metabolic Disorders, Historical Trends (since 2021) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.3 Peptides Market for Metabolic Disorders, Historical Trends (since 2021) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.4 Glass Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.5 Plastic Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.6 Single Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.7 Dual Chamber Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.8 Staked Needle Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.9 Luer Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.10 Disposable Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.11 Reusable Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.12 Conventional Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.13 Safety Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.14 Bulk Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.15 Nested Prefilled Syringes Market for Metabolic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.16 Prefilled Syringes Market for Metabolic Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.17 Prefilled Syringes Market for Metabolic Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.18 Prefilled Syringes Market for Metabolic Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.19 Prefilled Syringes Market for Metabolic Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 42.20 Prefilled Syringes Market for Metabolic Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.1 Proteins Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.2 Antibodies Market for Ophthalmic Disorders, Historical Trends (since 2020) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.3 Glass Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.4 Plastic Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.5 Single Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.6 Dual Chamber Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.7 Staked Needle Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.8 Luer Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.9 Disposable Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.10 Reusable Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.11 Conventional Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.12 Safety Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.13 Bulk Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.14 Nested Prefilled Syringes Market for Ophthalmic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.15 Prefilled Syringes Market for Ophthalmic Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.16 Prefilled Syringes Market for Ophthalmic Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.17 Prefilled Syringes Market for Ophthalmic Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.18 Prefilled Syringes Market for Ophthalmic Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 43.19 Prefilled Syringes Market for Ophthalmic Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.1 Antibodies Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.2 Small Molecules Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.3 Cell Therapies Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.4 Glass Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.5 Plastic Barrel Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.6 Single Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.7 Dual Chamber Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.8 Staked Needle Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.9 Luer Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.10 Disposable Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.11 Reusable Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.12 Conventional Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.13 Safety Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.14 Bulk Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.15 Nested Prefilled Syringes Market for Orthopedic Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.16 Prefilled Syringes Market for Orthopedic Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.17 Prefilled Syringes Market for Orthopedic Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.18 Prefilled Syringes Market for Orthopedic Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.19 Prefilled Syringes Market for Orthopedic Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 44.20 Prefilled Syringes Market for Orthopedic Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.1 Proteins Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.2 Antibodies Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.3 Peptides Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.4 Small Molecules Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.5 Vaccines Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.6 Cell Therapies Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.7 Glass Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.8 Plastic Barrel Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.9 Single Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.10 Dual Chamber Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.11 Staked Needle Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.12 Luer Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.13 Disposable Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.14 Reusable Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.15 Conventional Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.16 Safety Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.17 Bulk Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.18 Nested Prefilled Syringes Market for Other Disorders, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.19 Prefilled Syringes Market for Other Disorders in North America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.20 Prefilled Syringes Market for Other Disorders in Europe, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.21 Prefilled Syringes Market for Other Disorders in Asia-Pacific, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.22 Prefilled Syringes Market for Other Disorders in Middle East and North Africa, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 45.23 Prefilled Syringes Market for Other Disorders in Latin America, Historical Trends (since 2018) and Forecasted Estimates (till 2035) (USD Million)
- Figure 46.1 West Pharmaceutical Services: Annual Revenues, FY 2015 Onwards (USD Million)
- Figure 46.2 West Pharmaceutical Services: Distribution of Revenues by Business Divisions, FY 2022
- Figure 46.3 Datwyler: Annual Revenues, FY 2015 Onwards (CHF Billion)
- Figure 46.4 Aptar Pharma: Annual Revenues, FY 2015 Onwards (USD Billion)
- Figure 46.5 Stevanato: Annual Revenues, FY 2020 Onwards (EUR Million)
- Figure 46.6 Stevanato: Distribution of Revenues by Business Divisions, FY 2022
- Figure 47.1 Prefilled Syringe: Steps Involved in Fill / Finish Process
- Figure 47.2 Prefilled Syringe Fill / Finish Service Providers: Distribution by Year of Establishment
- Figure 47.3 Prefilled Syringe Fill / Finish Service Providers: Distribution by Location of Headquarters and Type of Drug Molecule Filled
- Figure 47.4 Prefilled Syringe Fill / Finish Service Providers: Distribution by Scale of Operation
- Figure 48.1 Autoject Micro: Mechanism of Drug Delivery
- Figure 48.2 Autoject 2 Autoinjector: Mechanism of Drug Delivery
- Figure 48.3 Autoject Mini: Mechanism of Drug Delivery
- Figure 48.4 Elcam Medical: Product Portfolio
- Figure 48.5 Flexi-Q PFS: Components
- Figure 48.6 Flexi-Q PFS: Mechanism of Drug Delivery
- Figure 48.7 Flexi-Q DV: Components
- Figure 48.8 Flexi-Q DV: Mechanism of Drug Delivery
- Figure 48.9 Flexi-Q EAI: Mechanism of Drug Delivery
- Figure 48.10 Flexi-Q mMU: Components
- Figure 48.11 Flexi-Q eMU: Components
- Figure 48.12 Flexi-Q mMU / Flexi-Q eMU: Mechanism of Drug Delivery
- Figure 48.13 Union Medico: 45°Autoinjector Portfolio
- Figure 48.14 45°/R Autoinjector: Components
- Figure 48.15 Types of 45°/R Autoinjectors
- Figure 48.16 Types of 90° Autoinjectors
- Figure 48.17 Exclusive Autoinjectors: Components
- Figure 48.18 SHL Medical: Product Portfolio
- Figure 48.19 SHL Medical: Prefilled Syringe based Autoinjectors
- Figure 48.20 SHL Medical: Cartridge based Autoinjectors
- Figure 48.21 SHL Medical: Two-Step Autoinjectors
- Figure 48.22 SHL Medical Two-Step Autoinjectors: Mechanism of Drug Delivery
- Figure 48.23 Ypsomed: Annual Revenues, FY 2014 Onwards (CHF Million)
- Figure 48.24 Ypsomed: Sales by Business Divisions, 2022 (CHF Million)
- Figure 48.25 YpsoMate: Mechanism of Drug Delivery
- Figure 48.26 VarioJect: Mechanism of Drug Delivery
- Figure 49.1 Concluding Remarks: Prefilled Syringes Market Landscape
- Figure 49.2 Concluding Remarks: Prefilled Syringe Combination Products Market Landscape
- Figure 49.3 Concluding Remarks: Recent Developments
- Figure 49.4 Concluding Remarks: Patent Analysis
- Figure 49.5 Concluding Remarks: KOL Analysis
- Figure 49.6 Concluding Remarks: Market Forecast and Opportunity Analysis (I/III)
- Figure 49.7 Concluding Remarks: Market Forecast and Opportunity Analysis (II/III)
- Figure 49.8 Concluding Remarks: Market Forecast and Opportunity Analysis (III/III)




