PUBLISHER: Roots Analysis | PRODUCT CODE: 1771291

PUBLISHER: Roots Analysis | PRODUCT CODE: 1771291
At Home Testing Kits Market: Industry Trends and Global Forecasts - Distribution by Type of Test Format, Type of Biofluid Analyzed, Therapeutic Area and Key Geographical Regions (North America, Europe, Asia-Pacific, Africa and South America )
GLOBAL AT HOME TESTING KITS MARKET: OVERVIEW
As per Roots Analysis, the global at home testing kits market is estimated to grow from USD 14.8 billion in the current year to USD 31.15 billion by 2035, at a CAGR of 6% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Test Format
- Strip
- Stick
- Cassette
- Others
Type of Biofluid Analyzed
- Urine
- Blood
- Stool
Therapeutic Area
- Fertility and Reproductive Health
- Metabolic Disorders
- Infectious Diseases
- Oncological Disorders
Area of Application
- Diagnostics
- Research
- Therapeutics
- Other Areas of Applications
Key Geographical Regions
- North America (US, Canada and Rest of North America)
- Europe (UK, Germany, France, Italy, Spain and Rest of Europe)
- Asia Pacific (India, China, Japan, South Korea, Indonesia and Rest of Asia-Pacific)
- Africa (Nigeria, Ethiopia, Egypt and Rest of Africa)
- South America (Argentina, Brazil and Rest of South America)
GLOBAL AT HOME TESTING KITS MARKET: GROWTH AND TRENDS
In the healthcare industry, digitization has evolved over time with numerous advancements, ensuring equitable access to novel health products and medical devices. Self- testing kits can improve health equity and accelerate progress towards universal health coverage, and it has the ability to improve health care. Self-testing kits provide individuals and communities with simple, quick, and private means to diagnose and treat a wide range of diseases, including HIV, hepatitis C, COVID-19, and, most recently, syphilis. People can now direct their own illness screening and make decisions based on their results safely, effectively, securely, and conveniently owing to more accessible and affordable self-tests and the expanding self-care movement.
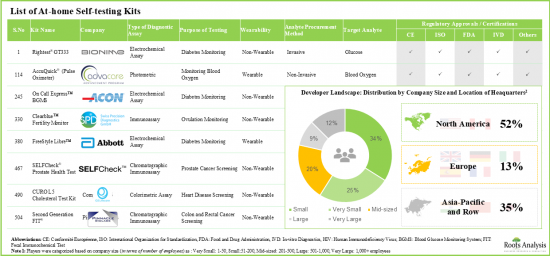
Owing to several advantages offered by at-home self-testing kits for diagnostics and screening purposes, industry stakeholders have undertaken numerous R&D initiatives focused on exploiting the use of such kits to provide healthcare at a fraction of a cost to the general public when compared to traditional clinics. Associated cost saving and privacy are main drivers of the growth of this domain over the coming decade. Currently, more than 650 at-home self-testing kits are available worldwide and are approved by regulatory authorities. The market is highly fragmented, featuring the presence of both new entrants and established players, based in different geographical regions.
GLOBAL AT HOME TESTING KITS MARKET: KEY INSIGHTS
The report delves into the current state of global at home testing kits market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Over 510 at-home self-test kits have been developed for the screening, monitoring and diagnosis of a myriad of diseases, including diabetes, colon cancer and HIV.
- Majority of the at-home self-testing strips are primarily being used for monitoring diabetes and have received approval from regulatory authorities, such as the USFDA, ISO and IVD.
- Presently, more than 51% of the total at-home self-testing kit developers are based in North America.
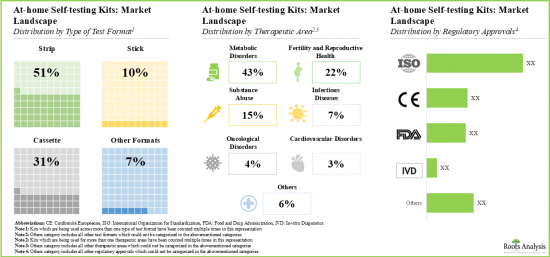
- It is worth noting that most of the at-home self-testing kits are currently being used for monitoring disorders (43%), followed by those employed for detection of conditions related to fertility and reproductive health (22%).
- Most of the kits are being developed to follow invasive route for the procurement of analyte (56%), followed by those being manufactured to utilize non-invasive methods for procuring analyte (43%).
- More than 1,130 academic grants have been awarded to industry players to support the ongoing efforts related to research and innovation of at-home self-testing kits, over the past few years.
- Several non-profit organizations have extended financial support to aid the research related to at-home self-testing kits. It is worth mentioning that NIAID emerged as the most prominent non-profit organization, having awarded over 23% of grants for a period of 1-5 years, followed by NHLBI (12%).
- In order to gain competitive edge, firms continue to improve and benchmark their existing capabilities and capacities to offer a number of at-home self-testing solutions.
- Cost is a key determinant of the acceptance and adoption of assay kits; the pricing strategy matrix is likely to assist players in evaluating the competitive market prices for their offerings.
- Due to competitive pricing of products and rapid innovation being led by companies in this market, there are various lucrative opportunities for long term investments, with high possibility of positive return on investments.
- The current capacity is adequate to satisfy global annual demand; however, we expect that industry players will continue to invest in additional capacity to address long-term growth and rising future demand.
- It is worth highlighting that the demand for HIV screening kits is expected to grow at a rate of 13%. Further, the highest demand for such products is expected to be generated by the Asia-Pacific region in 2035 (56%).
- In the long-term, the overall projected opportunity is likely to be segregated across various therapeutic areas, biofluids analyzed, test formats and key geographical regions.
- The current market is driven by kits developed for metabolic disorders (64%); this trend is unlikely to change in the foreseen future as well. Further, based on type of test format, strip-based tests are likely to have the maximum revenue generation potential (50%), by 2035.
Example Players in the At Home Testing Kits Market
- ACON Laboratories,
- AdvaCare Pharma USA
- Apex Biotechnology
- i-SENS
- Oak Tree Health
- TaiDoc Technology
- VivaCheck Laboratories
GLOBAL At Home Testing Kits Market: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global antiviral drugs market, focusing on key market segments, including [A] type of test format, [B] type of biofluid analyzed, [C] therapeutic area, [D] area of application and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies engaged in the development of at-home self-testing kits, considering various parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of testing procedure, [E] type of test format, [F] purpose of testing, [G] target therapeutic area, [H] wearability, [I] analyte procurement method, [J] biofluid analyzed, [K] processing time, [L] kit components, [M] regulatory approvals / certifications, [N] kit reusability, [O] availability of data management tools, [P] target analyte detected and [P] follow-up consultation requirement.
- Grant Analysis: An in-depth analysis of academic grants that have been awarded to various research institutes for conducting research focused on at-home self-testing kits, based on various relevant parameters, such as [A] year of grant award, [B] amount awarded, [C] support period, [D] type of funding institute center, [E] grant application, [F] purpose of grant award, [G] grant activity code, [H] NIH spending category, [I] study section involved, [J] popular NIH departments, [K] recipient organization, [L] regional distribution of recipient organization.
- Company Competitiveness Analysis: A comprehensive competitive analysis of at-home self-testing kit manufacturers, examining factors, such as [A] overall experience of the company, [B] product portfolio strength and [C] portfolio diversity.
- Company Profiles: In-depth profiles of companies engaged in offering at-home self-testing kits, focusing on [A] company overviews, [B] financial information (if available), [C] product portfolio and [D] recent developments and an informed future outlook.
- Bowman Clock Pricing Strategy: A comprehensive assessment to understand the pricing strategy of the at home self-testing kits offered by a firm, along with its competitive position in the market.
- Company Comparable Analysis: An in-depth analysis of company's intrinsic value by using comparable company analysis of publicly listed companies based on various parameters, such as [A] their current share price, [B] equity value, [C] enterprise value, [D] EBITDA, [E] net income and [F] revenue.
- Demand Analysis: A comprehensive analysis of annual demand for at-home self-testing kits, across various therapeutic areas, such as [A]] fertility and reproductive health, [B] metabolic disorders, [C] infectious diseases and [D] oncological disorders.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
- 2.1. Chapter Overview
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to At-Home Self-Testing
- 3.3. Types of At-home Tests
- 3.4. Types of At-home Test Assays
- 3.5. Advantages and Disadvantages Associated with At-home Self-testing Kits
- 3.6. Regulations Established for At-home Self-testing Kits
- 3.7. Future Perspective
4. MARKET OVERVIEW
- 4.1. Chapter Overview
- 4.2. At-home Self-testing Kits: List of Developers
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Company Size and Location of Headquarters
- 4.3. At-home Self-testing Kits: Overall Market Landscape
- 4.3.1. Analysis by Type of Testing Procedure
- 4.3.2. Analysis by Type of Test Format
- 4.3.3. Analysis by Purpose of Testing
- 4.3.4. Analysis by Target Therapeutic Area
- 4.3.5. Analysis by Kit Components
- 4.3.6. Analysis by Kit Wearability
- 4.3.7. Analysis by Target Analyte Detected
- 4.3.8. Analysis by Type of Analyte Procurement Method
- 4.3.9. Analysis by Type of Biofluid Analyzed
- 4.3.10. Analysis by Processing Time
- 4.3.11. Analysis by Number of Regulatory Approvals
- 4.3.12. Analysis by Kit Reusability
- 4.3.13. Analysis by Availability of Data Management Tools
- 4.3.14. Analysis by Follow-up Consultation Requirement
5. GRANTS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Scope and Methodology
- 5.3. At-home Self-testing Kits: Grant Analysis
- 5.3.1. Analysis by Year of Grant Award
- 5.3.2. Analysis by Amount Awarded
- 5.3.3. Analysis by Support Period
- 5.3.4. Analysis by Support Period and Funding Institute Center
- 5.3.5. Analysis by Type of Grant Application
- 5.3.6. Analysis by Purpose of Grant Award
- 5.3.7. Analysis by Activity Code
- 5.3.8. Analysis by NIH Spending Categories (Word Cloud Representation)
- 5.3.9. Analysis by Study Section Involved
- 5.3.10. Popular NIH Departments: Analysis by Number of Grants
- 5.3.11. Analysis by Type of Recipient Organization
- 5.3.12. Prominent Program Officers: Analysis by Number of Grants
- 5.3.13. Popular Recipient Organizations: Analysis by Number of Grants
- 5.3.14. Popular Recipient Organizations: Analysis by Amount Awarded
- 5.3.15. Analysis by Region of Recipient Organization
6. COMPANY COMPETITIVENESS ANALYSIS
- 6.1. Chapter Overview
- 6.2. Key Assumptions and Parameters
- 6.3. Methodology
- 6.4. At-home Self-testing Kit Developers: Company Competitiveness Analysis
- 6.4.1. At-home Self-testing Kits Developers Based in North America
- 6.4.2. At-home Self-testing Kits Developers Based in Europe
- 6.4.3. At-home Self-testing Kits Developers Based in Asia-Pacific and Rest of the World
- 6.4.4. At-home Self-testing Kits Developers: Competitive Benchmarking
7. COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. ACON Laboratories
- 7.2.1. Company Overview
- 7.2.2. Product Portfolio
- 7.2.3. Recent Developments and Future Outlook
- 7.3. AdvaCare Pharma USA
- 7.3.1. Company Overview
- 7.3.2. Product Portfolio
- 7.3.3. Recent Developments and Future Outlook
- 7.4. Apex Biotechnology
- 7.4.1. Company Overview
- 7.4.2. Product Portfolio
- 7.4.3. Recent Developments and Future Outlook
- 7.5. i-SENS
- 7.5.1. Company Overview
- 7.5.2. Financial Information
- 7.5.3. Product Portfolio
- 7.5.4. Recent Developments and Future Outlook
- 7.6. Oak Tree Health
- 7.6.1. Company Overview
- 7.6.2. Product Portfolio
- 7.6.3. Recent Developments and Future Outlook
- 7.7. TaiDoc Technology
- 7.7.1. Company Overview
- 7.7.2. Product Portfolio
- 7.7.3. Recent Developments and Future Outlook
- 7.8. VivaCheck Laboratories
- 7.8.1. Company Overview
- 7.8.2. Product Portfolio
- 7.8.3. Recent Developments and Future Outlook
8. BOWMAN CLOCK PRICING STRATEGY
- 8.1. Chapter Overview
- 8.2. Bowman Strategy Clock
- 8.2.1. Two Dimensions of Bowman Strategy Clock
- 8.2.2. Eight Positions on Bowman Strategy Clock
- 8.3. Roots Analysis Framework
- 8.3.1. Methodology
- 8.3.2. Theoretical Framework and Price Evaluation Hypothesis
- 8.3.3. Results and Interpretation
- 8.3.3.1. Product Price Evaluation Matrix: Information on Type of Diagnostic Assay
- 8.3.3.2. Product Price Evaluation Matrix: Information on Temperature
- 8.3.3.3. Product Price Evaluation Matrix: Information on Purpose of Testing
- 8.3.3.4. Product Price Evaluation Matrix: Information on Type of Target Analyte
- 8.3.3.5. Product Price Evaluation Matrix: Information on Type of Test Format
- 8.4. Concluding Remarks
9. COMPANY COMPARABLE ANALYSIS
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. Operating Statistics for Comparable Companies
- 9.3.1. Operating Statistics: Abbott Laboratories
- 9.3.2. Operating Statistics: Roche
- 9.3.3. Operating Statistics: Johnson & Johnson
- 9.3.4. Operating Statistics: Median, 25th And 75th Percentile Multiple
- 9.4. Valuation Statistics for Comparable Companies
- 9.4.1. Valuation Statistics: Abbott Laboratories
- 9.4.2. Valuation Statistics: Roche
- 9.4.3. Valuation Statistics: Johnson & Johnson
- 9.4.4. Valuation Statistics: Median, 25th And 75th Percentile Multiple
- 9.5. Valuation Summary for Abbott Laboratories
- 9.5.1. Valuation Summary: Implied Share Price from Discount Cash Flow
- 9.5.2. Football Field Analysis: Abbott Laboratories
- 9.6. Valuation Summary for Roche
- 9.6.1. Valuation Summary: Implied Share Price from Discount Cash Flow
- 9.6.2. Football Field Analysis: Roche
- 9.7. Valuation Summary for Johnson & Johnson
- 9.7.1. Valuation Summary: Implied Share Price from Discount Cash Flow
- 9.7.2. Football Field Analysis: Johnson & Johnson
10. DEMAND ANALYSIS
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Global Demand for At-home Self-testing Kits
- 10.4. Demand for At-home Self-testing Kits: Analysis by Geography
- 10.5. Demand for At-home Self-testing Kits: Analysis by Therapeutic Area
- 10.5.1. Demand for At-home Self-testing Fertility and Reproductive Health Kits
- 10.5.1.1. Demand for At-home Self-testing Pregnancy Test Kits
- 10.5.1.1.1. Demand for At-home Self-testing Pregnancy Test Kits in North America
- 10.5.1.1.1.1. Demand for At-home Self-testing Pregnancy Test Kits in the US
- 10.5.1.1.1.2. Demand for At-home Self-testing Pregnancy Test Kits in Canada
- 10.5.1.1.1.3. Demand for At-home Self-testing Pregnancy Test Kits in Rest of North America
- 10.5.1.1.2. Demand for At-home Self-testing Pregnancy Test Kits in Europe
- 10.5.1.1.2.1. Demand for At-home Self-testing Pregnancy Test Kits in the UK
- 10.5.1.1.2.2. Demand for At-home Self-testing Pregnancy Test Kits in Germany
- 10.5.1.1.2.3. Demand for At-home Self-testing Pregnancy Test Kits in Spain
- 10.5.1.1.2.4. Demand for At-home Self-testing Pregnancy Test Kits in Italy
- 10.5.1.1.2.5. Demand for At-home Self-testing Pregnancy Test Kits in France
- 10.5.1.1.2.6. Demand for At-home Self-testing Pregnancy Test Kits in Rest of Europe
- 10.5.1.1.3. Demand for At-home Self-testing Pregnancy Test Kits in Asia-Pacific
- 10.5.1.1.3.1. Demand for At-home Self-testing Pregnancy Test Kits in India
- 10.5.1.1.3.2. Demand for At-home Self-testing Pregnancy Test Kits in China
- 10.5.1.1.3.3. Demand for At-home Self-testing Pregnancy Test Kits in Japan
- 10.5.1.1.3.4. Demand for At-home Self-testing Pregnancy Test Kits in South Korea
- 10.5.1.1.3.5. Demand for At-home Self-testing Pregnancy Test Kits in Indonesia
- 10.5.1.1.3.6. Demand for At-home Self-testing Pregnancy Test Kits in Rest of Asia-Pacific
- 10.5.1.1.4. Demand for At-home Self-testing Pregnancy Test Kits in Africa
- 10.5.1.1.4.1. Demand for At-home Self-testing Pregnancy Test Kits in Nigeria
- 10.5.1.1.4.2. Demand for At-home Self-testing Pregnancy Test Kits in Ethiopia
- 10.5.1.1.4.3. Demand for At-home Self-testing Pregnancy Test Kits in Egypt
- 10.5.1.1.4.4. Demand for At-home Self-testing Pregnancy Test Kits in Rest of Africa
- 10.5.1.1.5. Demand for At-home Self-testing Pregnancy Test Kits in South America
- 10.5.1.1.5.1. Demand for At-home Self-testing Pregnancy Test Kits in Argentina
- 10.5.1.1.5.2. Demand for At-home Self-testing Pregnancy Test Kits in Brazil
- 10.5.1.1.5.3. Demand for At-home Self-testing Pregnancy Test Kits in Rest of South America
- 10.5.1.1.1. Demand for At-home Self-testing Pregnancy Test Kits in North America
- 10.5.1.2. Demand for At-home Self-testing Ovulation Test Kits
- 10.5.1.2.1. Demand for At-home Self-testing Ovulation Test Kits in North America
- 10.5.1.2.1.1. Demand for At-home Self-testing Ovulation Test Kits in the US
- 10.5.1.2.1.2. Demand for At-home Self-testing Ovulation Test Kits in Canada
- 10.5.1.2.1.3. Demand for At-home Self-testing Ovulation Test Kits in Rest of North America
- 10.5.1.2.2. Demand for At-home Self-testing Ovulation Test Kits in Europe
- 10.5.1.2.2.1. Demand for At-home Self-testing Ovulation Test Kits in the UK
- 10.5.1.2.2.2. Demand for At-home Self-testing Ovulation Test Kits in Germany
- 10.5.1.2.2.3. Demand for At-home Self-testing Ovulation Test Kits in Spain
- 10.5.1.2.2.4. Demand for At-home Self-testing Ovulation Test Kits in Italy
- 10.5.1.2.2.5. Demand for At-home Self-testing Ovulation Test Kits in France
- 10.5.1.2.2.6. Demand for At-home Self-testing Ovulation Test Kits in Rest of Europe
- 10.5.1.2.3. Demand for At-home Self-testing Ovulation Test Kits in Asia-Pacific
- 10.5.1.2.3.1. Demand for At-home Self-testing Ovulation Test Kits in India
- 10.5.1.2.3.2. Demand for At-home Self-testing Ovulation Test Kits in China
- 10.5.1.2.3.3. Demand for At-home Self-testing Ovulation Test Kits in Japan
- 10.5.1.2.3.4. Demand for At-home Self-testing Ovulation Test Kits in South Korea
- 10.5.1.2.3.5. Demand for At-home Self-testing Ovulation Test Kits in Indonesia
- 10.5.1.2.3.6. Demand for At-home Self-testing Ovulation Test Kits in Rest of Asia-Pacific
- 10.5.1.2.4. Demand for At-home Self-testing Ovulation Test Kits in Africa
- 10.5.1.2.4.1. Demand for At-home Self-testing Ovulation Test Kits in Nigeria
- 10.5.1.2.4.2. Demand for At-home Self-testing Ovulation Test Kits in Ethiopia
- 10.5.1.2.4.3. Demand for At-home Self-testing Ovulation Test Kits in Egypt
- 10.5.1.2.4.4. Demand for At-home Self-testing Ovulation Test Kits in Rest of Africa
- 10.5.1.2.5. Demand for At-home Self-testing Ovulation Test Kits in South America
- 10.5.1.2.5.1. Demand for At-home Self-testing Ovulation Test Kits in Argentina
- 10.5.1.2.5.2. Demand for At-home Self-testing Ovulation Test Kits in Brazil
- 10.5.1.2.5.3. Demand for At-home Self-testing Ovulation Test Kits in Rest of South America
- 10.5.1.2.1. Demand for At-home Self-testing Ovulation Test Kits in North America
- 10.5.1.1. Demand for At-home Self-testing Pregnancy Test Kits
- 10.5.2. Demand for At-home Self-testing Infectious Diseases Kits
- 10.5.2.1. Demand for At-home Self-testing HIV Test Kits
- 10.5.2.1.1. Demand for At-home Self-testing HIV Test Kits in North America
- 10.5.2.1.1.1. Demand for At-home Self-testing HIV Test Kits in the US
- 10.5.2.1.1.2. Demand for At-home Self-testing HIV Test Kits in Canada
- 10.5.2.1.1.3. Demand for At-home Self-testing HIV Test Kits in Rest of North America
- 10.5.2.1.2. Demand for At-home Self-testing HIV Test Kits in Europe
- 10.5.2.1.2.1. Demand for At-home Self-testing HIV Test Kits in the UK
- 10.5.2.1.2.2. Demand for At-home Self-testing HIV Test Kits in Germany
- 10.5.2.1.2.3. Demand for At-home Self-testing HIV Test Kits in Spain
- 10.5.2.1.2.4. Demand for At-home Self-testing HIV Test Kits in Italy
- 10.5.2.1.2.5. Demand for At-home Self-testing HIV Test Kits in France
- 10.5.2.1.2.6. Demand for At-home Self-testing HIV Test Kits in Rest of Europe
- 10.5.2.1.3. Demand for At-home Self-testing HIV Test Kits in Asia-Pacific
- 10.5.2.1.3.1. Demand for At-home Self-testing HIV Test Kits in India
- 10.5.2.1.3.2. Demand for At-home Self-testing HIV Test Kits in China
- 10.5.2.1.3.3. Demand for At-home Self-testing HIV Test Kits in Japan
- 10.5.2.1.3.4. Demand for At-home Self-testing HIV Test Kits in South Korea
- 10.5.2.1.3.5. Demand for At-home Self-testing HIV Test Kits in Indonesia
- 10.5.2.1.3.6. Demand for At-home Self-testing HIV Test Kits in Rest of Asia-Pacific
- 10.5.2.1.4. Demand for At-home Self-testing HIV Test Kits in Africa
- 10.5.2.1.4.1. Demand for At-home Self-testing HIV Test Kits in Nigeria
- 10.5.2.1.4.2. Demand for At-home Self-testing HIV Test Kits in Ethiopia
- 10.5.2.1.4.3. Demand for At-home Self-testing HIV Test Kits in Egypt
- 10.5.2.1.4.4. Demand for At-home Self-testing HIV Test Kits in Rest of Africa
- 10.5.2.1.5. Demand for At-home Self-testing HIV Test Kits in South America
- 10.5.2.1.5.1. Demand for At-home Self-testing HIV Test Kits in Argentina
- 10.5.2.1.5.2. Demand for At-home Self-testing HIV Test Kits in Brazil
- 10.5.2.1.5.3. Demand for At-home Self-testing HIV Test Kits in Rest of South America
- 10.5.2.1.1. Demand for At-home Self-testing HIV Test Kits in North America
- 10.5.2.1. Demand for At-home Self-testing HIV Test Kits
- 10.5.3. Demand for At-home Self-testing Metabolic Disorders Kits
- 10.5.3.1. Demand for At-home Self-testing Diabetes Test Kits
- 10.5.3.1.1. Demand for At-home Self-testing Diabetes Test Kits in North America
- 10.5.3.1.1.1. Demand for At-home Self-testing Diabetes Test Kits in the US
- 10.5.3.1.1.2. Demand for At-home Self-testing Diabetes Test Kits in Canada
- 10.5.3.1.1.3. Demand for At-home Self-testing Diabetes Test Kits in Rest of North America
- 10.5.3.1.2. Demand for At-home Self-testing Diabetes Test Kits in Europe
- 10.5.3.1.2.1. Demand for At-home Self-testing Diabetes Test Kits in the UK
- 10.5.3.1.2.2. Demand for At-home Self-testing Diabetes Test Kits in Germany
- 10.5.3.1.2.3. Demand for At-home Self-testing Diabetes Test Kits in Spain
- 10.5.3.1.2.4. Demand for At-home Self-testing Diabetes Test Kits in Italy
- 10.5.3.1.2.5. Demand for At-home Self-testing Diabetes Test Kits in France
- 10.5.3.1.2.6. Demand for At-home Self-testing Diabetes Test Kits in Rest of Europe
- 10.5.3.1.3. Demand for At-home Self-testing Diabetes Test Kits in Asia-Pacific
- 10.5.3.1.3.1. Demand for At-home Self-testing Diabetes Test Kits in India
- 10.5.3.1.3.2. Demand for At-home Self-testing Diabetes Test Kits in China
- 10.5.3.1.3.3. Demand for At-home Self-testing Diabetes Test Kits in Japan
- 10.5.3.1.3.4. Demand for At-home Self-testing Diabetes Test Kits in South Korea
- 10.5.3.1.3.5. Demand for At-home Self-testing Diabetes Test Kits in Indonesia
- 10.5.3.1.3.6. Demand for At-home Self-testing Diabetes Test Kits in Rest of Asia-Pacific
- 10.5.3.1.4. Demand for At-home Self-testing Diabetes Test Kits in Africa
- 10.5.3.1.4.1. Demand for At-home Self-testing Diabetes Test Kits in Nigeria
- 10.5.3.1.4.2. Demand for At-home Self-testing Diabetes Test Kits in Ethiopia
- 10.5.3.1.4.3. Demand for At-home Self-testing Diabetes Test Kits in Egypt
- 10.5.3.1.4.4. Demand for At-home Self-testing Diabetes Test Kits in Rest of Africa
- 10.5.3.1.5. Demand for At-home Self-testing Diabetes Test Kits in South America
- 10.5.3.1.5.1. Demand for At-home Self-testing Diabetes Test Kits in Argentina
- 10.5.3.1.5.2. Demand for At-home Self-testing Diabetes Test Kits in Brazil
- 10.5.3.1.5.3. Demand for At-home Self-testing Diabetes Test Kits in Rest of South America
- 10.5.3.1.1. Demand for At-home Self-testing Diabetes Test Kits in North America
- 10.5.3.1. Demand for At-home Self-testing Diabetes Test Kits
- 10.5.4. Demand for At-home Self-testing Oncological Disorders Kits
- 10.5.4.1. Demand for At-home Self-testing Colon Cancer Test Kits
- 10.5.4.1.1. Demand for At-home Self-testing Colon Cancer Test Kits in North America
- 10.5.4.1.1.1. Demand for At-home Self-testing Colon Cancer Test Kits in the US
- 10.5.4.1.1.2. Demand for At-home Self-testing Colon Cancer Test Kits in Canada
- 10.5.4.1.1.3. Demand for At-home Self-testing Colon Cancer Test Kits in Rest of North America
- 10.5.4.1.2. Demand for At-home Self-testing Colon Cancer Test Kits in Europe
- 10.5.4.1.2.1. Demand for At-home Self-testing Colon Cancer Test Kits in the UK
- 10.5.4.1.2.2. Demand for At-home Self-testing Colon Cancer Test Kits in Germany
- 10.5.4.1.2.3. Demand for At-home Self-testing Colon Cancer Test Kits in Spain
- 10.5.4.1.2.4. Demand for At-home Self-testing Colon Cancer Test Kits in Italy
- 10.5.4.1.2.5. Demand for At-home Self-testing Colon Cancer Test Kits in France
- 10.5.4.1.2.6. Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Europe
- 10.5.4.1.3. Demand for At-home Self-testing Colon Cancer Test Kits in Asia-Pacific
- 10.5.4.1.3.1. Demand for At-home Self-testing Colon Cancer Test Kits in India
- 10.5.4.1.3.2. Demand for At-home Self-testing Colon Cancer Test Kits in China
- 10.5.4.1.3.3. Demand for At-home Self-testing Colon Cancer Test Kits in Japan
- 10.5.4.1.3.4. Demand for At-home Self-testing Colon Cancer Test Kits in South Korea
- 10.5.4.1.3.5. Demand for At-home Self-testing Colon Cancer Test Kits in Indonesia
- 10.5.4.1.3.6. Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Asia-Pacific
- 10.5.4.1.4. Demand for At-home Self-testing Colon Cancer Test Kits in Africa
- 10.5.4.1.4.1. Demand for At-home Self-testing Colon Cancer Test Kits in Nigeria
- 10.5.4.1.4.2. Demand for At-home Self-testing Colon Cancer Test Kits in Ethiopia
- 10.5.4.1.4.3. Demand for At-home Self-testing Colon Cancer Test Kits in Egypt
- 10.5.4.1.4.4. Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Africa
- 10.5.4.1.5. Demand for At-home Self-testing Colon Cancer Test Kits in South America
- 10.5.4.1.5.1. Demand for At-home Self-testing Colon Cancer Test Kits in Argentina
- 10.5.4.1.5.2. Demand for At-home Self-testing Colon Cancer Test Kits in Brazil
- 10.5.4.1.5.3. Demand for At-home Self-testing Colon Cancer Test Kits in Rest of South America
- 10.5.4.1.1. Demand for At-home Self-testing Colon Cancer Test Kits in North America
- 10.5.4.1. Demand for At-home Self-testing Colon Cancer Test Kits
- 10.5.1. Demand for At-home Self-testing Fertility and Reproductive Health Kits
- 10.6. Concluding Remarks
11. MARKET FORECAST AND OPPUTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Global At-home Self-testing Kits Market, Till 2035
- 11.4. At-home Self-testing Kits Market: Analysis by Geography
- 11.5. At-home Self-testing Kits Market: Analysis by Therapeutic Area, Till 2035
- 11.5.1. At-home Self-testing Kits Market for Fertility and Reproductive Health, Till 2035
- 11.5.1.1. At-home Self-testing Kits Market for Pregnancy Detection, Till 2035
- 11.5.1.1.1. At-home Self-testing Kits Market for Pregnancy Detection in North America, Till 2035
- 11.5.1.1.1.1. At-home Self-testing Kits Market for Pregnancy Detection in the US, Till 2035
- 11.5.1.1.1.2. At-home Self-testing Kits Market for Pregnancy Detection in Canada, Till 2035
- 11.5.1.1.1.3. At-home Self-testing Kits Market for Pregnancy Detection in Rest of North America, Till 2035
- 11.5.1.1.2. At-home Self-testing Kits Market for Pregnancy Detection in Europe, Till 2035
- 11.5.1.1.2.1. At-home Self-testing Kits Market for Pregnancy Detection in the UK, Till 2035
- 11.5.1.1.2.2. At-home Self-testing Kits Market for Pregnancy Detection in Germany, Till 2035
- 11.5.1.1.2.3. At-home Self-testing Kits Market for Pregnancy Detection in Spain, Till 2035
- 11.5.1.1.2.4. At-home Self-testing Kits Market for Pregnancy Detection in Italy, Till 2035
- 11.5.1.1.2.5. At-home Self-testing Kits Market for Pregnancy Detection in France, Till 2035
- 11.5.1.1.2.6. At-home Self-testing Kits Market for Pregnancy Detection in Rest of Europe, Till 2035
- 11.5.1.1.3. At-home Self-testing Kits Market for Pregnancy Detection in Asia-Pacific, Till 2035
- 11.5.1.1.3.1. At-home Self-testing Kits Market for Pregnancy Detection in India, Till 2035
- 11.5.1.1.3.2. At-home Self-testing Kits Market for Pregnancy Detection in China, Till 2035
- 11.5.1.1.3.3. At-home Self-testing Kits Market for Pregnancy Detection in Japan, Till 2035
- 11.5.1.1.3.4. At-home Self-testing Kits Market for Pregnancy Detection in South Korea, Till 2035
- 11.5.1.1.3.5. At-home Self-testing Kits Market for Pregnancy Detection in Indonesia, Till 2035
- 11.5.1.1.3.6. At-home Self-testing Kits Market for Pregnancy Detection in Rest of Asia-Pacific, Till 2035
- 11.5.1.1.4. At-home Self-testing Kits Market for Pregnancy Detection in Africa, Till 2035
- 11.5.1.1.4.1. At-home Self-testing Kits Market for Pregnancy Detection in Nigeria, Till 2035
- 11.5.1.1.4.2. At-home Self-testing Kits Market for Pregnancy Detection in Ethiopia, Till 2035
- 11.5.1.1.4.3. At-home Self-testing Kits Market for Pregnancy Detection in Egypt, Till 2035
- 11.5.1.1.4.4. At-home Self-testing Kits Market for Pregnancy Detection in Rest of Africa, Till 2035
- 11.5.1.1.5. At-home Self-testing Kits Market for Pregnancy Detection in South America, Till 2035
- 11.5.1.1.5.1. At-home Self-testing Kits Market for Pregnancy Detection in Argentina, Till 2035
- 11.5.1.1.5.2. At-home Self-testing Kits Market for Pregnancy Detection in Brazil, Till 2035
- 11.5.1.1.5.3. At-home Self-testing Kits Market for Pregnancy Detection in Rest of South America, Till 2035
- 11.5.1.1.1. At-home Self-testing Kits Market for Pregnancy Detection in North America, Till 2035
- 11.5.1.2. At-home Self-testing Kits Market for Ovulation Monitoring, Till 2035
- 11.5.1.2.1. At-home Self-testing Kits Market for Ovulation Monitoring in North America, Till 2035
- 11.5.1.2.1.1. At-home Self-testing Kits Market for Ovulation Monitoring in the US, Till 2035
- 11.5.1.2.1.2. At-home Self-testing Kits Market for Ovulation Monitoring in Canada, Till 2035
- 11.5.1.2.1.3. At-home Self-testing Kits Market for Ovulation Monitoring in Rest of North America, Till 2035
- 11.5.1.2.2. At-home Self-testing Kits Market for Ovulation Monitoring in Europe, Till 2035
- 11.5.1.2.2.1. At-home Self-testing Kits Market for Ovulation Monitoring in the UK, Till 2035
- 11.5.1.2.2.2. At-home Self-testing Kits Market for Ovulation Monitoring in Germany, Till 2035
- 11.5.1.2.2.3. At-home Self-testing Kits Market for Ovulation Monitoring in Spain, Till 2035
- 11.5.1.2.2.4. At-home Self-testing Kits Market for Ovulation Monitoring in Italy, Till 2035
- 11.5.1.2.2.5. At-home Self-testing Kits Market for Ovulation Monitoring in France, Till 2035
- 11.5.1.2.2.6. At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Europe, Till 2035
- 11.5.1.2.3. At-home Self-testing Kits Market for Ovulation Monitoring in Asia-Pacific, Till 2035
- 11.5.1.2.3.1. At-home Self-testing Kits Market for Ovulation Monitoring in India, Till 2035
- 11.5.1.2.3.2. At-home Self-testing Kits Market for Ovulation Monitoring in China, Till 2035
- 11.5.1.2.3.3. At-home Self-testing Kits Market for Ovulation Monitoring in Japan, Till 2035
- 11.5.1.2.3.4. At-home Self-testing Kits Market for Ovulation Monitoring in South Korea, Till 2035
- 11.5.1.2.3.5. At-home Self-testing Kits Market for Ovulation Monitoring in Indonesia, Till 2035
- 11.5.1.2.3.6. At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Asia-Pacific, Till 2035
- 11.5.1.2.4. At-home Self-testing Kits Market for Ovulation Monitoring in Africa, Till 2035
- 11.5.1.2.4.1. At-home Self-testing Kits Market for Ovulation Monitoring in Nigeria, Till 2035
- 11.5.1.2.4.2. At-home Self-testing Kits Market for Ovulation Monitoring in Ethiopia, Till 2035
- 11.5.1.2.4.3. At-home Self-testing Kits Market for Ovulation Monitoring in Egypt, Till 2035
- 11.5.1.2.4.4. At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Africa, Till 2035
- 11.5.1.2.5. At-home Self-testing Kits Market for Ovulation Monitoring in South America, Till 2035
- 11.5.1.2.5.1. At-home Self-testing Kits Market for Ovulation Monitoring in Argentina, Till 2035
- 11.5.1.2.5.2. At-home Self-testing Kits Market for Ovulation Monitoring in Brazil, Till 2035
- 11.5.1.2.5.3. At-home Self-testing Kits Market for Ovulation Monitoring in Rest of South America, Till 2035
- 11.5.1.2.1. At-home Self-testing Kits Market for Ovulation Monitoring in North America, Till 2035
- 11.5.1.1. At-home Self-testing Kits Market for Pregnancy Detection, Till 2035
- 11.5.2. At-home Self-testing Kits Market for Infectious Diseases, Till 2035
- 11.5.2.1. At-home Self-testing Kits Market for HIV Detection, Till 2035
- 11.5.2.1.1. At-home Self-testing Kits Market for HIV Detection in North America, Till 2035
- 11.5.2.1.1.1. At-home Self-testing Kits Market for HIV Detection in the US, Till 2035
- 11.5.2.1.1.2. At-home Self-testing Kits Market for HIV Detection in Canada, Till 2035
- 11.5.2.1.1.3. At-home Self-testing Kits Market for HIV Detection in Rest of North America, Till 2035
- 11.5.2.1.2. At-home Self-testing Kits Market for HIV Detection in Europe, Till 2035
- 11.5.2.1.2.1. At-home Self-testing Kits Market for HIV Detection in the UK, Till 2035
- 11.5.2.1.2.2. At-home Self-testing Kits Market for HIV Detection in Germany, Till 2035
- 11.5.2.1.2.3. At-home Self-testing Kits Market for HIV Detection in Spain, Till 2035
- 11.5.2.1.2.4. At-home Self-testing Kits Market for HIV Detection in Italy, Till 2035
- 11.5.2.1.2.5. At-home Self-testing Kits Market for HIV Detection in France, Till 2035
- 11.5.2.1.2.6. At-home Self-testing Kits Market for HIV Detection in Rest of Europe, Till 2035
- 11.5.2.1.3. At-home Self-testing Kits Market for HIV Detection in Asia-Pacific, Till 2035
- 11.5.2.1.3.1. At-home Self-testing Kits Market for HIV Detection in India, Till 2035
- 11.5.2.1.3.2. At-home Self-testing Kits Market for HIV Detection in China, Till 2035
- 11.5.2.1.3.3. At-home Self-testing Kits Market for HIV Detection in Japan, Till 2035
- 11.5.2.1.3.4. At-home Self-testing Kits Market for HIV Detection in South Korea, Till 2035
- 11.5.2.1.3.5. At-home Self-testing Kits Market for HIV Detection in Indonesia, Till 2035
- 11.5.2.1.3.6. At-home Self-testing Kits Market for HIV Detection in Rest of Asia-Pacific, Till 2035
- 11.5.2.1.4. At-home Self-testing Kits Market for HIV Detection in Africa, Till 2035
- 11.5.2.1.4.1. At-home Self-testing Kits Market for HIV Detection in Nigeria, Till 2035
- 11.5.2.1.4.2. At-home Self-testing Kits Market for HIV Detection in Ethiopia, Till 2035
- 11.5.2.1.4.3. At-home Self-testing Kits Market for HIV Detection in Egypt, Till 2035
- 11.5.2.1.4.4. At-home Self-testing Kits Market for HIV Detection in Rest of Africa, Till 2035
- 11.5.2.1.5. At-home Self-testing Kits Market for HIV Detection in South America, Till 2035
- 11.5.2.1.5.1. At-home Self-testing Kits Market for HIV Detection in Argentina, Till 2035
- 11.5.2.1.5.2. At-home Self-testing Kits Market for HIV Detection in Brazil, Till 2035
- 11.5.2.1.5.3. At-home Self-testing Kits Market for HIV Detection in Rest of South America, Till 2035
- 11.5.2.1.1. At-home Self-testing Kits Market for HIV Detection in North America, Till 2035
- 11.5.2.1. At-home Self-testing Kits Market for HIV Detection, Till 2035
- 11.5.3. At-home Self-testing Kits Market for Metabolic Disorders, Till 2035
- 11.5.3.1. At-home Self-testing Kits Market for Diabetes Monitoring, Till 2035
- 11.5.3.1.1. At-home Self-testing Kits Market for Diabetes Monitoring in North America, Till 2035
- 11.5.3.1.1.1. At-home Self-testing Kits Market for Diabetes Monitoring in the US, Till 2035
- 11.5.3.1.1.2. At-home Self-testing Kits Market for Diabetes Monitoring in Canada, Till 2035
- 11.5.3.1.1.3. At-home Self-testing Kits Market for Diabetes Monitoring in Rest of North America, Till 2035
- 11.5.3.1.2. At-home Self-testing Kits Market for Diabetes Monitoring in Europe, Till 2035
- 11.5.3.1.2.1. At-home Self-testing Kits Market for Diabetes Monitoring in the UK, Till 2035
- 11.5.3.1.2.2. At-home Self-testing Kits Market for Diabetes Monitoring in Germany, Till 2035
- 11.5.3.1.2.3. At-home Self-testing Kits Market for Diabetes Monitoring in Spain, Till 2035
- 11.5.3.1.2.4. At-home Self-testing Kits Market for Diabetes Monitoring in Italy, Till 2035
- 11.5.3.1.2.5. At-home Self-testing Kits Market for Diabetes Monitoring in France, Till 2035
- 11.5.3.1.2.6. At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Europe, Till 2035
- 11.5.3.1.3. At-home Self-testing Kits Market for Diabetes Monitoring in Asia-Pacific, Till 2035
- 11.5.3.1.3.1. At-home Self-testing Kits Market for Diabetes Monitoring in India, Till 2035
- 11.5.3.1.3.2. At-home Self-testing Kits Market for Diabetes Monitoring in China, Till 2035
- 11.5.3.1.3.3. At-home Self-testing Kits Market for Diabetes Monitoring in Japan, Till 2035
- 11.5.3.1.3.4. At-home Self-testing Kits Market for Diabetes Monitoring in South Korea, Till 2035
- 11.5.3.1.3.5. At-home Self-testing Kits Market for Diabetes Monitoring in Indonesia, Till 2035
- 11.5.3.1.3.6. At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Asia-Pacific, Till 2035
- 11.5.3.1.4. At-home Self-testing Kits Market for Diabetes Monitoring in Africa, Till 2035
- 11.5.3.1.4.1. At-home Self-testing Kits Market for Diabetes Monitoring in Nigeria, Till 2035
- 11.5.3.1.4.2. At-home Self-testing Kits Market for Diabetes Monitoring in Ethiopia, Till 2035
- 11.5.3.1.4.3. At-home Self-testing Kits Market for Diabetes Monitoring in Egypt, Till 2035
- 11.5.3.1.4.4. At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Africa, Till 2035
- 11.5.3.1.5. At-home Self-testing Kits Market for Diabetes Monitoring in South America, Till 2035
- 11.5.3.1.5.1. At-home Self-testing Kits Market for Diabetes Monitoring in Argentina, Till 2035
- 11.5.3.1.5.2. At-home Self-testing Kits Market for Diabetes Monitoring in Brazil, Till 2035
- 11.5.3.1.5.3. At-home Self-testing Kits Market for Diabetes Monitoring in Rest of South America, Till 2035
- 11.5.3.1.1. At-home Self-testing Kits Market for Diabetes Monitoring in North America, Till 2035
- 11.5.3.1. At-home Self-testing Kits Market for Diabetes Monitoring, Till 2035
- 11.5.4. At-home Self-testing Kits Market for Oncological Disorders, Till 2035
- 11.5.4.1. At-home Self-testing Kits Market for Colon Cancer Detection, Till 2035
- 11.5.4.1.1. At-home Self-testing Kits Market for Colon Cancer Detection in North America, Till 2035
- 11.5.4.1.1.1. At-home Self-testing Kits Market for Colon Cancer Detection in the US, Till 2035
- 11.5.4.1.1.2. At-home Self-testing Kits Market for Colon Cancer Detection in Canada, Till 2035
- 11.5.4.1.1.3. At-home Self-testing Kits Market for Colon Cancer Detection in Rest of North America, Till 2035
- 11.5.4.1.2. At-home Self-testing Kits Market for Colon Cancer Detection in Europe, Till 2035
- 11.5.4.1.2.1. At-home Self-testing Kits Market for Colon Cancer Detection in the UK, Till 2035
- 11.5.4.1.2.2. At-home Self-testing Kits Market for Colon Cancer Detection in Germany, Till 2035
- 11.5.4.1.2.3. At-home Self-testing Kits Market for Colon Cancer Detection in Spain, Till 2035
- 11.5.4.1.2.4. At-home Self-testing Kits Market for Colon Cancer Detection in Italy, Till 2035
- 11.5.4.1.2.5. At-home Self-testing Kits Market for Colon Cancer Detection in France, Till 2035
- 11.5.4.1.2.6. At-home Self-testing Kits Market for Colon Cancer Detection in Rest of Europe, Till 2035
- 11.5.4.1.3. At-home Self-testing Kits Market for Colon Cancer Detection in Asia-Pacific, Till 2035
- 11.5.4.1.3.1. At-home Self-testing Kits Market for Colon Cancer Detection in India, Till 2035
- 11.5.4.1.3.2. At-home Self-testing Kits Market for Colon Cancer Detection in China, Till 2035
- 11.5.4.1.3.3. At-home Self-testing Kits Market for Colon Cancer Detection in Japan, Till 2035
- 11.5.4.1.3.4. At-home Self-testing Kits Market for Colon Cancer Detection in South Korea, Till 2035
- 11.5.4.1.3.5. At-home Self-testing Kits Market for Colon Cancer Detection in Indonesia, Till 2035
- 11.5.4.1.3.6. At-home Self-testing Kits Market for Colon Cancer Detection in Rest of Asia-Pacific, Till 2035
- 11.5.4.1.4. At-home Self-testing Kits Market for Colon Cancer Detection in Africa, Till 2035
- 11.5.4.1.4.1. At-home Self-testing Kits Market for Colon Cancer Detection in Nigeria, Till 2035
- 11.5.4.1.4.2. At-home Self-testing Kits Market for Colon Cancer Detection in Ethiopia, Till 2035
- 11.5.4.1.4.3. At-home Self-testing Kits Market for Colon Cancer Detection in Egypt, Till 2035
- 11.5.4.1.4.4. At-home Self-testing Kits Market for Colon Cancer Detection in Rest of Africa, Till 2035
- 11.5.4.1.5. At-home Self-testing Kits Market for Colon Cancer Detection in South America, Till 2035
- 11.5.4.1.5.1. At-home Self-testing Kits Market for Colon Cancer Detection in Argentina, Till 2035
- 11.5.4.1.5.2. At-home Self-testing Kits Market for Colon Cancer Detection in Brazil, Till 2035
- 11.5.4.1.5.3. At-home Self-testing Kits Market for Colon Cancer Detection in Rest of South America
- 11.5.4.1.1. At-home Self-testing Kits Market for Colon Cancer Detection in North America, Till 2035
- 11.5.4.1. At-home Self-testing Kits Market for Colon Cancer Detection, Till 2035
- 11.5.1. At-home Self-testing Kits Market for Fertility and Reproductive Health, Till 2035
- 11.6. At-home Self-testing Kits Market: Analysis by Type of Test Format, Till 2035
- 11.6.1. At-home Self-testing Kits Market for Stick based Tests, Till 2035
- 11.6.2. At-home Self-testing Kits Market for Strip based Tests, Till 2035
- 11.6.3. At-home Self-testing Kits Market for Cassette based Tests, Till 2035
- 11.6.4. At-home Self-testing Kits Market for Other Formats, Till 2035
- 11.7. At-home Self-testing Kits Market: Analysis by Type of Biofluid Analyzed, Till 2035
- 11.7.1. At-home Self-testing Kits Market for Urine based Tests, Till 2035
- 11.7.2. At-home Self-testing Kits Market for Blood based Tests, Till 2035
- 11.7.3. At-home Self-testing Kits Market for Stool based Tests, Till 2035
12. ATTRACTIVENESS COMPETETIVENESS MATRIX
- 12.1. Chapter Overview
- 12.2. AC Matrix: An Overview
- 12.2.1. Strong Business Segments
- 12.2.2. Average Business Segments
- 12.2.3. Weak Business Segments
- 12.3. Analytical Methodology
- 12.4. AC Matrix: Overall At-home Self-testing Kits Market Scenario
- 12.4.1. AC Matrix: Overall At-home Self-testing Kits Market Scenario for Target Therapeutic Area
- 12.4.2. AC Matrix: Overall At-home Self-testing Kits Market Scenario for Regions
13. CONCLUDING REMARKS
14. INTERVIEW TRANSCRIPTS
- 14.1. Chapter Overview
- 14.2. Company A
- 14.2.1. Company Snapshot
- 14.2.2. Interview Transcript, Vice President, Sales, and Marketing
- 14.3. Company B
- 14.3.1. Company Snapshot
- 14.3.2. Interview Transcript, Associate Vice President
15. APPENDIX I: TABULATED DATA
16. APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Information on Various Regulatory Authorities
- Table 4.1 List of At-home Self-testing Kit Developers
- Table 4.2 At-home Self-testing Kits: Information on Type of Testing Procedure and Type of Test Format
- Table 4.3 At-home Self-testing Kits: Information on Purpose of Testing and Target Therapeutic Area
- Table 4.4 At-home Self-testing Kits: Information on Kit Components and Kit Wearability
- Table 4.5 At-home Self-testing Kits: Information on Target Analyte Detected, Type of Analyte Procurement Method, and Type of Biofluid Analyzed
- Table 4.6 At-home Self-testing Kits: Information on Processing Time, Number of Regulatory Approvals and Kit Reusability
- Table 4.7 At-home Self-testing Kits: Information on Availability of Data Management Tools and Follow-up Consultation Requirement
- Table 7.1 Leading At-home Self-testing Kit Developers
- Table 7.2 ACON Laboratories: Company Overview
- Table 7.3 ACON Laboratories: Product Portfolio
- Table 7.4 ACON Laboratories: Recent Developments and Future Outlook
- Table 7.5 AdvaCare Pharma USA: Company Overview
- Table 7.6 AdvaCare Pharma USA: Product Portfolio
- Table 7.7 ApexBio: Company Overview
- Table 7.8 ApexBio: Product Portfolio
- Table 7.9 i-SENS: Company Overview
- Table 7.10 i-SENS: Product Portfolio
- Table 7.11 Oak Tree Health: Company Overview
- Table 7.12 Oak Tree Health: Product Portfolio
- Table 7.13 TaiDoc Technology: Company Overview
- Table 7.14 TaiDoc Technology: Product Portfolio
- Table 7.15 VivaChek Laboratories: Company Overview
- Table 7.16 VivaChek Laboratories: Product Portfolio
- Table 7.17 VivaChek Laboratories: Recent Developments and Future Outlook
- Table 8.1 Product Price Evaluation Matrix: Information on Information on Type of Diagnostic Assay (USD)
- Table 8.2 Product Price Evaluation Matrix: Information on Temperature (USD)
- Table 8.3 Product Price Evaluation Matrix: Information on Purpose of Testing (USD)
- Table 8.4 Product Price Evaluation Matrix: Information on Target Analyte (USD)
- Table 8.5 Product Price Evaluation Matrix: Type of Test Format (USD)
- Table 9.1 Operating Statistics: Abbott Laboratories
- Table 9.2 Operating Statistics: Roche
- Table 9.3 Operating Statistics: Johnson & Johnson
- Table 9.4 Operating Statistics: Median, 25th and 75th Percentile Multiple
- Table 9.5 Optimal Ranges of the Multiples
- Table 9.6 Valuation Statistics: Abbott Laboratories
- Table 9.7 Valuation Statistics: Roche
- Table 9.8 Valuation Statistics: Johnson and Johnson
- Table 9.9 Valuation Summary for Abbott Laboratories: Implied Enterprise and Equity Value (USD Million)
- Table 9.10 Valuation Summary for Abbott Laboratories: Implied Share Price based on Enterprise Value
- Table 9.11 Valuation Summary for Roche: Implied Enterprise and Equity Value (USD Million)
- Table 9.12 Valuation Summary for Roche: Implied Share Price based on Enterprise Value
- Table 9.13 Valuation Summary for Johnson and Johnson: Implied Enterprise and Equity Value (USD Million)
- Table 9.14 Valuation Summary for Johnson and Johnson: Implied Share Price based on Enterprise Value
- Table 10.1 Demand Analysis: Information on Market Maturity based on Target Indications and Geographical Locations
- Table 10.2 Demand Analysis: Information on Base Percentage Calculation
- Table 10.3 Demand Analysis: Information on Base Percentage for Therapeutic Area
- Table 15.1 At-home Self-testing Kit Developers: Distribution by Year of Establishment
- Table 15.2 At-home Self-testing Kit Developers: Distribution by Company Size
- Table 15.3 At-home Self-testing Kit Developers: Distribution by Location of Headquarters
- Table 15.4 At-home Self-testing Kit Developers: Distribution by Company Size and Location of Headquarters
- Table 15.5 At-home Self-testing Kits: Distribution by Type of Testing Procedure
- Table 15.6 At-home Self-testing Kits: Distribution by Type of Test Format
- Table 15.7 At-home Self-testing Kits: Distribution by Purpose of Testing
- Table 15.8 At-home Self-testing Kits: Distribution by Target Therapeutic Area
- Table 15.9 At-home Self-testing Kits: Distribution by Kit Components
- Table 15.10 At-home Self-testing Kits: Distribution by Kit Wearability
- Table 15.11 At-home Self-testing Kits: Distribution by Target Analyte Detected
- Table 15.12 At-home Self-testing Kits: Distribution by Type of Analyte Procurement Method
- Table 15.13 At-home Self-testing Kits: Distribution by Type of Biofluid Analyzed
- Table 15.14 At-home Self-testing Kits: Distribution by Number of Regulatory Approvals
- Table 15.15 At-home Self-testing Kits: Distribution by Kit Reusability
- Table 15.16 At-home Self-testing Kits: Distribution by Availability of Data Management Tools
- Table 15.17 At-home Self-testing Kits: Distribution by Follow-up Consultation Requirement
- Table 15.18 Grant Analysis: Cumulative Trend by Year of Grant, Since Pre-2019
- Table 15.19 Grant Analysis: Distribution by Amount Awarded (USD Million), Since Pre-2019
- Table 15.20 Grant Analysis: Distribution by Support Period
- Table 15.21 Grant Analysis: Distribution by Support Period and Funding Institute Center
- Table 15.22 Grant Analysis: Distribution by Type of Grant Application
- Table 15.23 Grant Analysis: Distribution by Purpose of Grant Award
- Table 15.24 Grant Analysis: Distribution by Study Section Involved
- Table 15.25 Popular NIH Departments: Distribution by Number of Grants
- Table 15.26 Grant Analysis: Distribution by Type of Recipient Organization
- Table 15.27 Prominent Program Officers: Distribution by Number of Grants
- Table 15.28 Popular Recipient Organization: Distribution by Number of Grants
- Table 15.29 Popular Recipient Organization: Distribution by Grant Amount (USD Million)
- Table 15.30 Grant Analysis: Distribution by Region of Recipient Organization
- Table 15.31 i-SENS: Annual Revenues (USD Million)
- Table 15.32 At-home Self-testing Kits: Bowman Clock Pricing Strategy Graphical Interpretation
- Table 15.33 Global Demand for At-home Self-testing Kits, Till 2035 (Million Units)
- Table 15.34 Demand for At-home Self-testing Kits: Distribution by Geography (Million Units)
- Table 15.35 Demand for At-home Self-testing Kits: Distribution by Target Therapeutic Area, Till 2035 (Million Units)
- Table 15.36 Demand for At-home Self-testing Pregnancy Test Kits, Till 2035 (Million Units)
- Table 15.37 Demand for At-home Self-testing Pregnancy Test Kits in North America, Till 2035 (Million Units)
- Table 15.38 Demand for At-home Self-testing Pregnancy Test Kits in the US, Till 2035 (Million Units)
- Table 15.39 Demand for At-home Self-testing Pregnancy Test Kits in Canada, Till 2035 (Million Units)
- Table 15.40 Demand for At-home Self-testing Pregnancy Test Kits in Rest of North America, Till 2035 (Million Units)
- Table 15.41 Demand for At-home Self-testing Pregnancy Test Kits in Europe, Till 2035 (Million Units)
- Table 15.42 Demand for At-home Self-testing Pregnancy Test Kits in the UK, Till 2035 (Million Units)
- Table 15.43 Demand for At-home Self-testing Pregnancy Test Kits in Germany, Till 2035 (Million Units)
- Table 15.44 Demand for At-home Self-testing Pregnancy Test Kits in Spain, Till 2035 (Million Units)
- Table 15.45 Demand for At-home Self-testing Pregnancy Test Kits in Italy, Till 2035 (Million Units)
- Table 15.46 Demand for At-home Self-testing Pregnancy Test Kits in France, Till 2035 (Million Units)
- Table 15.47 Demand for At-home Self-testing Pregnancy Test Kits in Rest of Europe, Till 2035 (Million Units)
- Table 15.48 Demand for At-home Self-testing Pregnancy Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Table 15.49 Demand for At-home Self-testing Pregnancy Test Kits in India, Till 2035 (Million Units)
- Table 15.50 Demand for At-home Self-testing Pregnancy Test Kits in China, Till 2035 (Million Units)
- Table 15.51 Demand for At-home Self-testing Pregnancy Test Kits in Japan, Till 2035 (Million Units)
- Table 15.52 Demand for At-home Self-testing Pregnancy Test Kits in South Korea, Till 2035 (Million Units)
- Table 15.53 Demand for At-home Self-testing Pregnancy Test Kits in Indonesia, Till 2035 (Million Units)
- Table 15.54 Demand for At-home Self-testing Pregnancy Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Table 15.55 Demand for At-home Self-testing Pregnancy Test Kits in Africa, Till 2035 (Million Units)
- Table 15.56 Demand for At-home Self-testing Pregnancy Test Kits in Nigeria, Till 2035 (Million Units)
- Table 15.57 Demand for At-home Self-testing Pregnancy Test Kits in Ethiopia, Till 2035 (Million Units)
- Table 15.58 Demand for At-home Self-testing Pregnancy Test Kits in Egypt, Till 2035 (Million Units)
- Table 15.59 Demand for At-home Self-testing Pregnancy Test Kits in Rest of Africa, Till 2035 (Million Units)
- Table 15.60 Demand for At-home Self-testing Pregnancy Test Kits in South America, Till 2035 (Million Units)
- Table 15.61 Demand for At-home Self-testing Pregnancy Test Kits in Argentina, Till 2035 (Million Units)
- Table 15.62 Demand for At-home Self-testing Pregnancy Test Kits in Brazil, Till 2035 (Million Units)
- Table 15.63 Demand for At-home Self- Pregnancy Test Kits in Rest of South America, Till 2035 (Million Units)
- Table 15.64 Demand for At-home Self-testing Ovulation Test Kits, Till 2035 (Million Units)
- Table 15.65 Demand for At-home Self-testing Ovulation Test Kits in North America, Till 2035 (Million Units)
- Table 15.66 Demand for At-home Self-testing Ovulation Test Kits in US, Till 2035 (Million Units)
- Table 15.67 Demand for At-home Self-testing Ovulation Test Kits in Canada, Till 2035 (Million Units)
- Table 15.68 Demand for At-home Self-testing Ovulation Test Kits in Rest of North America, Till 2035 (Million Units)
- Table 15.69 Demand for At-home Self-testing Ovulation Test Kits in Europe, Till 2035 (Million Units)
- Table 15.70 Demand for At-home Self-testing Ovulation Test Kits in UK, Till 2035 (Million Units)
- Table 15.71 Demand for At-home Self-testing Ovulation Test Kits in Germany, Till 2035 (Million Units)
- Table 15.72 Demand for At-home Self-testing Ovulation Test Kits in Spain, Till 2035 (Million Units)
- Table 15.73 Demand for At-home Self-testing Ovulation Test Kits in Italy, Till 2035 (Million Units)
- Table 15.74 Demand for At-home Self-testing Ovulation Test Kits in France, Till 2035 (Million Units)
- Table 15.75 Demand for At-home Self-testing Ovulation Test Kits in Rest of Europe, Till 2035 (Million Units)
- Table 15.76 Demand for At-home Self-testing Ovulation Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Table 15.77 Demand for At-home Self-testing Ovulation Test Kits in India, Till 2035 (Million Units)
- Table 15.78 Demand for At-home Self-testing Ovulation Test Kits in China, Till 2035 (Million Units)
- Table 15.79 Demand for At-home Self-testing Ovulation Test Kits in Japan, Till 2035 (Million Units)
- Table 15.80 Demand for At-home Self-testing Ovulation Test Kits in South Korea, Till 2035 (Million Units)
- Table 15.81 Demand for At-home Self-testing Ovulation Test Kits in Indonesia, Till 2035 (Million Units)
- Table 15.82 Demand for At-home Self-testing Ovulation Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Table 15.83 Demand for At-home Self-testing Ovulation Test Kits in Africa, Till 2035 (Million Units)
- Table 15.84 Demand for At-home Self-testing Ovulation Test Kits in Nigeria, Till 2035 (Million Units)
- Table 15.85 Demand for At-home Self-testing Ovulation Test Kits in Ethiopia, Till 2035 (Million Units)
- Table 15.86 Demand for At-home Self-testing Ovulation Test Kits in Egypt, Till 2035 (Million Units)
- Table 15.87 Demand for At-home Self-testing Ovulation Test Kits in Rest of Africa, Till 2035 (Million Units)
- Table 15.88 Demand for At-home Self-testing Ovulation Test Kits in South America, Till 2035 (Million Units)
- Table 15.89 Demand for At-home Self-testing Ovulation Test Kits in Argentina, Till 2035 (Million Units)
- Table 15.90 Demand for At-home Self-testing Ovulation Test Kits in Brazil, Till 2035 (Million Units)
- Table 15.91 Demand for At-home Self-testing Ovulation Test Kits in Rest of South America, Till 2035 (Million Units)
- Table 15.92 Demand for At-home Self-testing HIV Test Kits, Till 2035 (Million Units)
- Table 15.93 Demand for At-home Self-testing HIV Test Kits in North America, Till 2035 (Million Units)
- Table 15.94 Demand for At-home Self-testing HIV Test Kits in the US, Till 2035 (Million Units)
- Table 15.95 Demand for At-home Self-testing HIV Test Kits in Canada, Till 2035 (Million Units)
- Table 15.96 Demand for At-home Self-testing HIV Test Kits in Rest of North America, Till 2035 (Million Units)
- Table 15.97 Demand for At-home Self-testing HIV Test Kits in Europe, Till 2035 (Million Units)
- Table 15.98 Demand for At-home Self-testing HIV Test Kits in UK, Till 2035 (Million Units)
- Table 15.99 Demand for At-home Self-testing HIV Test Kits in Germany, Till 2035 (Million Units)
- Table 15.100 Demand for At-home Self-testing HIV Test Kits in Spain, Till 2035 (Million Units)
- Table 15.101 Demand for At-home Self-testing HIV Test Kits in Italy, Till 2035 (Million Units)
- Table 15.102 Demand for At-home Self-testing HIV Test Kits in France, Till 2035 (Million Units)
- Table 15.103 Demand for At-home Self-testing HIV Test Kits in Rest of Europe, Till 2035 (Million Units)
- Table 15.104 Demand for At-home Self-testing HIV Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Table 15.105 Demand for At-home Self-testing HIV Test Kits in India, Till 2035 (Million Units)
- Table 15.106 Demand for At-home Self-testing HIV Test Kits in China, Till 2035 (Million Units)
- Table 15.107 Demand for At-home Self-testing HIV Test Kits in Japan, Till 2035 (Million Units)
- Table 15.108 Demand for At-home Self-testing HIV Test Kits in South Korea, Till 2035 (Million Units)
- Table 15.109 Demand for At-home Self-testing HIV Test Kits in Indonesia, Till 2035 (Million Units)
- Table 15.110 Demand for At-home Self-testing HIV Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Table 15.111 Demand for At-home Self-testing Kits: Distribution of HIV Test Kits in Africa, Till 2035 (Million Units)
- Table 15.112 Demand for At-home Self-testing HIV Test Kits in Nigeria, Till 2035 (Million Units)
- Table 15.113 Demand for At-home Self-testing HIV Test Kits in Ethiopia, Till 2035 (Million Units)
- Table 15.114 Demand for At-home Self-testing HIV Test Kits in Egypt, Till 2035 (Million Units)
- Table 15.115 Demand for At-home Self-testing HIV Test Kits in Rest of Africa, Till 2035 (Million Units)
- Table 15.116 Demand for At-home Self-testing HIV Test Kits in South America, Till 2035 (Million Units)
- Table 15.117 Demand for At-home Self-testing HIV Test Kits in Argentina, Till 2035 (Million Units)
- Table 15.118 Demand for At-home Self-testing HIV Test Kits in Brazil, Till 2035 (Million Units)
- Table 15.119 Demand for At-home Self-testing HIV Test Kits in Rest of South America, Till 2035 (Million Units)
- Table 15.120 Demand for At-home Self-testing Diabetes Test Kits, Till 2035 (Million Units)
- Table 15.121 Demand for At-home Self-testing Diabetes Test Kits in North America, Till 2035 (Million Units)
- Table 15.122 Demand for At-home Self-testing Diabetes Test Kits in the US, Till 2035 (Million Units)
- Table 15.123 Demand for At-home Self-testing Diabetes Test Kits in Canada, Till 2035 (Million Units)
- Table 15.124 Demand for At-home Self-testing Diabetes Test Kits in Rest of North America, Till 2035 (Million Units)
- Table 15.125 Demand for At-home Self-testing Diabetes Test Kits in Europe, Till 2035 (Million Units)
- Table 15.126 Demand for At-home Self-testing Diabetes Test Kits in the UK, Till 2035 (Million Units)
- Table 15.127 Demand for At-home Self-testing Diabetes Test Kits in Germany, Till 2035 (Million Units)
- Table 15.128 Demand for At-home Self-testing Diabetes Test Kits in Spain, Till 2035 (Million Units)
- Table 15.129 Demand for At-home Self-testing Diabetes Test Kits in Italy, Till 2035 (Million Units)
- Table 15.130 Demand for At-home Self-testing Diabetes Test Kits in France, Till 2035 (Million Units)
- Table 15.131 Demand for At-home Self-testing Diabetes Test Kits in Rest of Europe, Till 2035 (Million Units)
- Table 15.132 Demand for At-home Self-testing Diabetes Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Table 15.133 Demand for At-home Self-testing Diabetes Test Kits in India, Till 2035 (Million Units)
- Table 15.134 Demand for At-home Self-testing Diabetes Test Kits in China, Till 2035 (Million Units)
- Table 15.135 Demand for At-home Self-testing Diabetes Test Kits in Japan, Till 2035 (Million Units)
- Table 15.136 Demand for At-home Self-testing Diabetes Test Kits in South Korea, Till 2035 (Million Units)
- Table 15.137 Demand for At-home Self-testing Diabetes Test Kits in Indonesia, Till 2035 (Million Units)
- Table 15.138 Demand for At-home Self-testing Diabetes Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Table 15.139 Demand for At-home Self-testing Diabetes Test Kits in Africa, Till 2035 (Million Units)
- Table 15.140 Demand for At-home Self-testing Diabetes Test Kits in Nigeria, Till 2035 (Million Units)
- Table 15.141 Demand for At-home Self-testing Diabetes Test Kits in Ethiopia, Till 2035 (Million Units)
- Table 15.142 Demand for At-home Self-testing Diabetes Test Kits in Egypt, Till 2035 (Million Units)
- Table 15.143 Demand for At-home Self-testing Diabetes Test Kits in Rest of Africa, Till 2035 (Million Units)
- Table 15.144 Demand for At-home Self-testing Diabetes Test Kits in South America, Till 2035 (Million Units)
- Table 15.145 Demand for At-home Self-testing Diabetes Test Kits in Argentina, Till 2035 (Million Units)
- Table 15.146 Demand for At-home Self-testing Diabetes Test Kits in Brazil, Till 2035 (Million Units)
- Table 15.147 Demand for At-home Self-testing Diabetes Test Kits in Rest of South America, Till 2035 (Million Units)
- Table 15.148 Demand for At-home Self-testing Colon Cancer Test Kits, Till 2035 (Million Units)
- Table 15.149 Demand for At-home Self-testing Colon Cancer Test Kits in North America, Till 2035 (Million Units)
- Table 15.150 Demand for At-home Self-testing Colon Cancer Test Kits in the US, Till 2035 (Million Units)
- Table 15.151 Demand for At-home Self-testing Colon Cancer Test Kits in Canada, Till 2035 (Million Units)
- Table 15.152 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of North America, Till 2035 (Million Units)
- Table 15.153 Demand for At-home Self-testing Colon Cancer Test Kits in Europe, Till 2035 (Million Units)
- Table 15.154 Demand for At-home Self-testing Colon Cancer Test Kits in the UK, Till 2035 (Million Units)
- Table 15.155 Demand for At-home Self-testing Colon Cancer Test Kits in Germany, Till 2035 (Million Units)
- Table 15.156 Demand for At-home Self-testing Colon Cancer Test Kits in Spain, Till 2035 (Million Units)
- Table 15.157 Demand for At-home Self-testing Colon Cancer Test Kits in Italy, Till 2035 (Million Units)
- Table 15.158 Demand for At-home Self-testing Colon Cancer Test Kits in France, Till 2035 (Million Units)
- Table 15.159 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Europe, Till 2035 (Million Units)
- Table 15.160 Demand for At-home Self-testing Colon Cancer Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Table 15.161 Demand for At-home Self-testing Colon Cancer Test Kits in India, Till 2035 (Million Units)
- Table 15.162 Demand for At-home Self-testing Colon Cancer Test Kits in China, Till 2035 (Million Units)
- Table 15.163 Demand for At-home Self-testing Colon Cancer Test Kits in Japan, Till 2035 (Million Units)
- Table 15.164 Demand for At-home Self-testing Colon Cancer Test Kits in South Korea, Till 2035 (Million Units)
- Table 15.165 Demand for At-home Self-testing Colon Cancer Test Kits in Indonesia, Till 2035 (Million Units)
- Table 15.166 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Table 15.167 Demand for At-home Self-testing Colon Cancer Test Kits in Africa, Till 2035 (Million Units)
- Table 15.168 Demand for At-home Self-testing Colon Cancer Test Kits in Nigeria, Till 2035 (Million Units)
- Table 15.169 Demand for At-home Self-testing Colon Cancer Test Kits in Ethiopia, Till 2035 (Million Units)
- Table 15.170 Demand for At-home Self-testing Colon Cancer Test Kits in Egypt, Till 2035 (Million Units)
- Table 15.171 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Africa, Till 2035 (Million Units)
- Table 15.172 Demand for At-home Self-testing Colon Cancer Test Kits in South America, Till 2035 (Million Units)
- Table 15.173 Demand for At-home Self-testing Colon Cancer Test Kits in Argentina, Till 2035 (Million Units)
- Table 15.174 Demand for At-home Self-testing Colon Cancer Test Kits in Brazil, Till 2035 (Million Units)
- Table 15.175 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of South America, Till 2035 (Million Units)
- Table 15.176 Global At-home Self-testing Kits Market, Till 2035 (USD Billion)
- Table 15.177 At-home Self-testing Kits Market: Distribution by Geography (USD Billion)
- Table 15.178 At-home Self-testing Kits Market: Distribution by Therapeutic Area, Till 2035 (USD Billion)
- Table 15.179 At-home Self-testing Kits Market for Pregnancy Detection, Till 2035 (USD Billion)
- Table 15.180 At-home Self-testing Kits Market: Distribution of Pregnancy Test Kits in North America, Till 2035 (USD Billion)
- Table 15.181 At-home Self-testing Kits Market for Pregnancy Detection in the US, Till 2035 (USD Billion)
- Table 15.182 At-home Self-testing Kits Market for Pregnancy Detection in Canada, Till 2035 (USD Billion)
- Table 15.183 At-home Self-testing Kits Market for Pregnancy Detection in Rest of North America, Till 2035 (USD Billion)
- Table 15.184 At-home Self-testing Kits Market for Pregnancy Detection in Europe, Till 2035 (USD Billion)
- Table 15.185 At-home Self-testing Kits Market for Pregnancy Detection in UK, Till 2035 (USD Billion)
- Table 15.186 At-home Self-testing Kits Market for Pregnancy Detection in Germany, Till 2035 (USD Billion)
- Table 15.187 At-home Self-testing Kits Market for Pregnancy Detection in Spain, Till 2035 (USD Billion)
- Table 15.188 At-home Self-testing Kits Market for Pregnancy Detection in Italy, Till 2035 (USD Billion)
- Table 15.189 At-home Self-testing Kits Market for Pregnancy Detection in France, Till 2035 (USD Billion)
- Table 15.190 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Europe, Till 2035 (USD Billion)
- Table 15.191 At-home Self-testing Kits Market for Pregnancy Detection in Asia-Pacific, Till 2035 (USD Billion)
- Table 15.192 At-home Self-testing Kits Market for Pregnancy Detection in India, Till 2035 (USD Billion)
- Table 15.193 At-home Self-testing Kits Market for Pregnancy Detection in China, Till 2035 (USD Billion)
- Table 15.194 At-home Self-testing Kits Market for Pregnancy Detection in Japan, Till 2035 (USD Billion)
- Table 15.195 At-home Self-testing Kits Market for Pregnancy Detection in South Korea, Till 2035 (USD Billion)
- Table 15.196 At-home Self-testing Kits Market for Pregnancy Detection in Indonesia, Till 2035 (USD Billion)
- Table 15.197 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Table 15.198 At-home Self-testing Kits Market for Pregnancy Detection in Africa, Till 2035 (USD Billion)
- Table 15.199 At-home Self-testing Kits Market for Pregnancy Detection in Nigeria, Till 2035 (USD Billion)
- Table 15.200 At-home Self-testing Kits Market for Pregnancy Detection in Ethiopia, Till 2035 (USD Billion)
- Table 15.201 At-home Self-testing Kits Market for Pregnancy Detection in Egypt, Till 2035 (USD Billion)
- Table 15.202 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Africa, Till 2035 (USD Billion)
- Table 15.203 At-home Self-testing Kits Market for Pregnancy Detection in South America, Till 2035 (USD Billion)
- Table 15.204 At-home Self-testing Kits Market for Pregnancy Detection in Argentina, Till 2035 (USD Billion)
- Table 15.205 At-home Self-testing Kits Market for Pregnancy Detection in Brazil, Till 2035 (USD Billion)
- Table 15.206 At-home Self-testing Kits Market for Pregnancy Detection in Rest of South America, Till 2035 (USD Billion)
- Table 15.207 At-home Self-testing Kits Market for Ovulation Monitoring, Till 2035 (USD Billion)
- Table 15.208 At-home Self-testing Kits Market: Distribution of Ovulation Test Kits in North America, Till 2035 (USD Billion)
- Table 15.209 At-home Self-testing Kits Market for Ovulation Monitoring in the US, Till 2035 (USD Billion)
- Table 15.210 At-home Self-testing Kits Market for Ovulation Monitoring in Canada, Till 2035 (USD Billion)
- Table 15.211 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of North America, Till 2035 (USD Billion)
- Table 15.212 At-home Self-testing Kits Market for Ovulation Monitoring in Europe, Till 2035 (USD Billion)
- Table 15.213 At-home Self-testing Kits Market for Ovulation Monitoring in UK, Till 2035 (USD Billion)
- Table 15.214 At-home Self-testing Kits Market for Ovulation Monitoring in Germany, Till 2035 (USD Billion)
- Table 15.215 At-home Self-testing Kits Market for Ovulation Monitoring in Spain, Till 2035 (USD Billion)
- Table 15.216 At-home Self-testing Kits Market for Ovulation Monitoring in Italy, Till 2035 (USD Billion)
- Table 15.217 At-home Self-testing Kits Market for Ovulation Monitoring in France, Till 2035 (USD Billion)
- Table 15.218 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Europe, Till 2035 (USD Billion)
- Table 15.219 At-home Self-testing Kits Market for Ovulation Monitoring in Asia-Pacific, Till 2035 (USD Billion)
- Table 15.220 At-home Self-testing Kits Market for Ovulation Monitoring in India, Till 2035 (USD Billion)
- Table 15.221 At-home Self-testing Kits Market for Ovulation Monitoring in China, Till 2035 (USD Billion)
- Table 15.222 At-home Self-testing Kits Market for Ovulation Monitoring in Japan, Till 2035 (USD Billion)
- Table 15.223 At-home Self-testing Kits Market for Ovulation Monitoring in South Korea, Till 2035 (USD Billion)
- Table 15.224 At-home Self-testing Kits Market for Ovulation Monitoring in Indonesia, Till 2035 (USD Billion)
- Table 15.225 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Table 15.226 At-home Self-testing Kits Market for Ovulation Monitoring in Africa, Till 2035 (USD Billion)
- Table 15.227 At-home Self-testing Kits Market for Ovulation Monitoring in Nigeria, Till 2035 (USD Billion)
- Table 15.228 At-home Self-testing Kits Market for Ovulation Monitoring in Ethiopia, Till 2035 (USD Billion)
- Table 15.229 At-home Self-testing Kits Market for Ovulation Monitoring in Egypt, Till 2035 (USD Billion)
- Table 15.230 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Africa, Till 2035 (USD Billion)
- Table 15.231 At-home Self-testing Kits Market for Ovulation Monitoring in South America, Till 2035 (USD Billion)
- Table 15.232 At-home Self-testing Kits Market for Ovulation Monitoring in Argentina, Till 2035 (USD Billion)
- Table 15.233 At-home Self-testing Kits Market for Ovulation Monitoring in Brazil, Till 2035 (USD Billion)
- Table 15.234 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of South America, Till 2035 (USD Billion)
- Table 15.235 At-home Self-testing Kits Market for HIV Detection, Till 2035 (USD Billion)
- Table 15.236 At-home Self-testing Kits Market: Distribution of HIV Test Kits in North America, Till 2035 (USD Billion)
- Table 15.237 At-home Self-testing Kits Market for HIV Detection in the US, Till 2035 (USD Billion)
- Table 15.239 At-home Self-testing Kits Market for HIV Detection in Canada, Till 2035 (USD Billion)
- Table 15.240 At-home Self-testing Kits Market for HIV Detection in Rest of North America, Till 2035 (USD Billion)
- Table 15.241 At-home Self-testing Kits Market for HIV Detection in Europe, Till 2035 (USD Billion)
- Table 15.242 At-home Self-testing Kits Market for HIV Detection in UK, Till 2035 (USD Billion)
- Table 15.243 At-home Self-testing Kits Market for HIV Detection in Germany, Till 2035 (USD Billion)
- Table 15.244 At-home Self-testing Kits Market for HIV Detection in Spain, Till 2035 (USD Billion)
- Table 15.245 At-home Self-testing Kits Market for HIV Detection in Italy, Till 2035 (USD Billion)
- Table 15.246 At-home Self-testing Kits Market for HIV Detection in France, Till 2035 (USD Billion)
- Table 15.247 At-home Self-testing Kits Market for HIV Detection in Rest of Europe, Till 2035 (USD Billion)
- Table 15.248 At-home Self-testing Kits Market for HIV Detection in Asia-Pacific, Till 2035 (USD Billion)
- Table 15.249 At-home Self-testing Kits Market for HIV Detection in India, Till 2035 (USD Billion)
- Table 15.250 At-home Self-testing Kits Market for HIV Detection in China, Till 2035 (USD Billion)
- Table 15.251 At-home Self-testing Kits Market for HIV Detection in Japan, Till 2035 (USD Billion)
- Table 15.252 At-home Self-testing Kits Market for HIV Detection in South Korea, Till 2035 (USD Billion)
- Table 15.253 At-home Self-testing Kits Market for HIV Detection in Indonesia, Till 2035 (USD Billion)
- Table 15.254 At-home Self-testing Kits Market for HIV Detection in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Table 15.255 At-home Self-testing Kits Market for HIV Detection in Africa, Till 2035 (USD Billion)
- Table 15.256 At-home Self-testing Kits Market for HIV Detection in Nigeria, Till 2035 (USD Billion)
- Table 15.257 At-home Self-testing Kits Market for HIV Detection in Ethiopia, Till 2035 (USD Billion)
- Table 15.258 At-home Self-testing Kits Market for HIV Detection in Egypt, Till 2035 (USD Billion)
- Table 15.259 At-home Self-testing Kits Market for HIV Detection in Rest of Africa, Till 2035 (USD Billion)
- Table 15.260 At-home Self-testing Kits Market for HIV Detection in South America, Till 2035 (USD Billion)
- Table 15.261 At-home Self-testing Kits Market for HIV Detection in Argentina, Till 2035 (USD Billion)
- Table 15.262 At-home Self-testing Kits Market for HIV Detection in Brazil, Till 2035 (USD Billion)
- Table 15.263 At-home Self-testing Kits Market for HIV Detection in Rest of South America, Till 2035 (USD Billion)
- Table 15.264 At-home Self-testing Kits Market for Diabetes Monitoring, Till 2035 (USD Billion)
- Table 15.265 At-home Self-testing Kits Market: Distribution of Diabetes Monitoring Test Kits in North America, Till 2035 (USD Billion)
- Table 15.266 At-home Self-testing Kits Market for Diabetes Monitoring in the US, Till 2035 (USD Billion)
- Table 15.267 At-home Self-testing Kits Market for Diabetes Monitoring in Canada, Till 2035 (USD Billion)
- Table 15.268 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of North America, Till 2035 (USD Billion)
- Table 15.269 At-home Self-testing Kits Market for Diabetes Monitoring in Europe, Till 2035 (USD Billion)
- Table 15.270 At-home Self-testing Kits Market for Diabetes Monitoring in UK, Till 2035 (USD Billion)
- Table 15.271 At-home Self-testing Kits Market for Diabetes Monitoring in Germany, Till 2035 (USD Billion)
- Table 15.272 At-home Self-testing Kits Market for Diabetes Monitoring in Spain, Till 2035 (USD Billion)
- Table 15.273 At-home Self-testing Kits Market for Diabetes Monitoring in Italy, Till 2035 (USD Billion)
- Table 15.274 At-home Self-testing Kits Market for Diabetes Monitoring in France, Till 2035 (USD Billion)
- Table 15.275 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Europe, Till 2035 (USD Billion)
- Table 15.276 At-home Self-testing Kits Market for Diabetes Monitoring in Asia-Pacific, Till 2035 (USD Billion)
- Table 15.277 At-home Self-testing Kits Market for Diabetes Monitoring in India, Till 2035 (USD Billion)
- Table 15.278 At-home Self-testing Kits Market for Diabetes Monitoring in China, Till 2035 (USD Billion)
- Table 15.279 At-home Self-testing Kits Market for Diabetes Monitoring in Japan, Till 2035 (USD Billion)
- Table 15.280 At-home Self-testing Kits Market for Diabetes Monitoring in South Korea, Till 2035 (USD Billion)
- Table 15.281 At-home Self-testing Kits Market for Diabetes Monitoring in Indonesia, Till 2035 (USD Billion)
- Table 15.282 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Table 15.283 At-home Self-testing Kits Market for Diabetes Monitoring in Africa, Till 2035 (USD Billion)
- Table 15.284 At-home Self-testing Kits Market for Diabetes Monitoring in Nigeria, Till 2035 (USD Billion)
- Table 15.285 At-home Self-testing Kits Market for Diabetes Monitoring in Ethiopia, Till 2035 (USD Billion)
- Table 15.286 At-home Self-testing Kits Market for Diabetes Monitoring in Egypt, Till 2035 (USD Billion)
- Table 15.287 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Africa, Till 2035 (USD Billion)
- Table 15.288 At-home Self-testing Kits Market for Diabetes Monitoring in South America, Till 2035 (USD Billion)
- Table 15.289 At-home Self-testing Kits Market for Diabetes Monitoring in Argentina, Till 2035 (USD Billion)
- Table 15.290 At-home Self-testing Kits Market for Diabetes Monitoring in Brazil, Till 2035 (USD Billion)
- Table 15.291 At-home Self-testing Kits Market for Diabetes Mon
List of Figures
Figure 2.1 Executive Summary: Market Overview
- Figure 2.2 Executive Summary: Grant Analysis
- Figure 2.3 Executive Summary: Demand Analysis
- Figure 2.4 Executive Summary: Market Forecast
- Figure 3.1 Key Steps Involved for Taking At-Home Test
- Figure 3.2 Advantages Offered by At-home Tests
- Figure 3.3 Disadvantages Associated with At-home Tests
- Figure 4.1 At-home Self-testing Kit Developers: Distribution by Year of Establishment
- Figure 4.2 At-home Self-testing Kit Developers: Distribution by Company Size
- Figure 4.3 At-home Self-testing Kit Developers: Distribution by Location of Headquarters
- Figure 4.4 At-home Self-testing Kit Developers: Distribution by Company Size and Location of Headquarters
- Figure 4.5 At-home Self-testing Kits: Distribution by Type of Testing Procedure
- Figure 4.6 At-home Self-testing Kits: Distribution by Type of Test Format
- Figure 4.7 At-home Self-testing Kits: Distribution by Purpose of Testing
- Figure 4.8 At-home Self-testing Kits: Distribution by Target Therapeutic Area
- Figure 4.9 At-home Self-testing Kits: Distribution by Kit Components
- Figure 4.10 At-home Self-testing Kits: Distribution by Kit Wearability
- Figure 4.11 At-home Self-testing Kits: Distribution by Target Analyte Detected
- Figure 4.12 At-home Self-testing Kits: Distribution by Type of Analyte Procurement Method
- Figure 4.13 At-home Self-testing Kits: Distribution by Type of Biofluid Analyzed
- Figure 4.14 At-home Self-testing Kits: Distribution by Number of Regulatory Approvals
- Figure 4.15 At-home Self-testing Kits: Distribution by Kit Reusability
- Figure 4.16 At-home Self-testing Kits: Distribution by Availability of Data Management Tools
- Figure 4.17 At-home Self-testing Kits: Distribution by Follow-up Consultation Requirement
- Figure 5.1 Grant Distribution: Cumulative Trend by Year of Grant, Since Pre-2019
- Figure 5.2 Grant Distribution: Distribution by Amount Awarded (USD Million), Since Pre-2019
- Figure 5.3 Grant Distribution: Distribution by Support Period
- Figure 5.4 Grant Distribution: Distribution by Support Period and Funding Institute Center
- Figure 5.5 Grant Distribution: Distribution by Type of Grant Application
- Figure 5.6 Grant Distribution: Distribution by Purpose of Grant Award
- Figure 5.7 Grant Distribution: Distribution by Activity Code
- Figure 5.8 Word Cloud: NIH Spending Categories
- Figure 5.9 Grant Distribution: Distribution by Study Section Involved
- Figure 5.10 Popular NIH Departments: Distribution by Number of Grants
- Figure 5.11 Grant Distribution: Distribution by Type of Recipient Organization
- Figure 5.12 Prominent Program Officers: Distribution by Number of Grants
- Figure 5.13 Popular Recipient Organization: Distribution by Number of Grants
- Figure 5.14 Popular Recipient Organization: Distribution by Amount Awarded
- Figure 5.15 Grant Distribution: Distribution by Region of Recipient Organization
- Figure 6.1 At-home Self-testing Kit Developers based in North America
- Figure 6.2 At-home Self-testing Kit Developers based in Europe
- Figure 6.3 At-home Self-testing Kit Developers based in Asia Pacific and Rest of the World
- Figure 6.4 Company Competitiveness Analysis: Competitiveness Benchmarking of At-home Self-testing Kit Developers
- Figure 7.1 i-SENS: Annual Revenues (USD Million)
- Figure 8.1 At-home Self-testing Kits (Metabolic Disorders): Bowman Clock Pricing Strategy Matrix
- Figure 8.2 At-home Self-testing Kits (Metabolic Disorders): Bowman Clock Pricing Strategy Graphical Interpretation
- Figure 9.1 At-home Self-testing Kits: Operation Statistics for Comparable Companies
- Figure 9.2 At-home Self-testing Kits: Valuation Statistics for Comparable Companies
- Figure 9.3 Spider Web Analysis for Abbott Laboratories: Implied Share Price based on Enterprise Value / Revenue and Enterprise Value / EBITDA
- Figure 9.4 Football Field Analysis: Abbott Laboratories
- Figure 9.5 Spider Web Analysis for Roche: Implied Share Price based on Enterprise Value / Revenue and Enterprise Value / EBITDA
- Figure 9.6 Football Field Analysis: Roche
- Figure 9.7 Spider Web Analysis for Johnson and Johnson: Implied Share Price based on Enterprise Value / Revenue and Enterprise Value / EBITDA
- Figure 9.8 Football Field Analysis: Johnson and Johnson
- Figure 10.1 Global Demand for At-home Self-testing Kits, Till 2035 (Million Units)
- Figure 10.2 At-home Self-testing Kits: Distribution by Geography (Million Units)
- Figure 10.3 At-home Self-testing Kits: Distribution by Therapeutic Area, Till 2035 (Million Units)
- Figure 10.4 Demand for At-home Self Testing Pregnancy Test Kits, Till 2035 (Million Units)
- Figure 10.5 Demand for At-home Self Testing Pregnancy Test Kits in North America, Till 2035 (Million Units)
- Figure 10.6 Demand for At-home Self Testing Pregnancy Test Kits in the US, Till 2035 (Million Units)
- Figure 10.7 Demand for At-home Self Testing Pregnancy Test Kits in Canada, Till 2035 (Million Units)
- Figure 10.8 Demand for At-home Self Testing Pregnancy Test Kits in Rest of North America, Till 2035 (Million Units)
- Figure 10.9 Demand for At-home Self Testing Pregnancy Test Kits in Europe, Till 2035 (Million Units)
- Figure 10.10 Demand for At-home Self Testing Pregnancy Test Kits in the UK, Till 2035 (Million Units)
- Figure 10.11 Demand for At-home Self Testing Pregnancy Test Kits in Germany, Till 2035 (Million Units)
- Figure 10.12 Demand for At-home Self Testing Pregnancy Test Kits in Spain, Till 2035 (Million Units)
- Figure 10.13 Demand for At-home Self Testing Pregnancy Test Kits in North Italy, Till 2035 (Million Units)
- Figure 10.14 Demand for At-home Self Testing Pregnancy Test Kits in France, Till 2035 (Million Units)
- Figure 10.15 Demand for At-home Self Testing Pregnancy Test Kits in Rest of Europe, Till 2035 (Million Units)
- Figure 10.16 Demand for At-home Self Testing Pregnancy Test Kits in Asia Pacific, Till 2035 (Million Units)
- Figure 10.17 Demand for At-home Self Testing Pregnancy Test Kits in India, Till 2035 (Million Units)
- Figure 10.18 Demand for At-home Self Testing Pregnancy Test Kits in China, Till 2035 (Million Units)
- Figure 10.19 Demand for At-home Self Testing Pregnancy Test Kits in Japan, Till 2035 (Million Units)
- Figure 10.20 Demand for At-home Self Testing Pregnancy Test Kits in South Korea, Till 2035 (Million Units)
- Figure 10.21 Demand for At-home Self Testing Pregnancy Test Kits in Indonesia, Till 2035 (Million Units)
- Figure 10.22 Demand for At-home Self Testing Pregnancy Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Figure 10.23 Demand for At-home Self Testing Pregnancy Test Kits in Africa, Till 2035 (Million Units)
- Figure 10.24 Demand for At-home Self Testing Pregnancy Test Kits in Nigeria, Till 2035 (Million Units)
- Figure 10.25 Demand for At-home Self Testing Pregnancy Test Kits in Ethiopia, Till 2035 (Million Units)
- Figure 10.26 Demand for At-home Self Testing Pregnancy Test Kits in Egypt, Till 2035 (Million Units)
- Figure 10.27 Demand for At-home Self Testing Pregnancy Test Kits in Rest of Africa, Till 2035 (Million Units)
- Figure 10.28 Demand for At-home Self Testing Pregnancy Test Kits in South America, Till 2035 (Million Units)
- Figure 10.29 Demand for At-home Self Testing Pregnancy Test Kits in Argentina, Till 2035 (Million Units)
- Figure 10.30 Demand for At-home Self Testing Pregnancy Test Kits in Brazil, Till 2035 (Million Units)
- Figure 10.31 Demand for At-home Self Testing Pregnancy Test Kits in Rest of South America, Till 2035 (Million Units)
- Figure 10.32 Demand for At-home Self Testing Ovulation Test Kits, Till 2035 (Million Units)
- Figure 10.33 Demand for At-home Self Testing Ovulation Test Kits in North America, Till 2035 (Million Units)
- Figure 10.34 Demand for At-home Self Testing Ovulation Test Kits in the US, Till 2035 (Million Units)
- Figure 10.35 Demand for At-home Self Testing Ovulation Test Kits in Canada, Till 2035 (Million Units)
- Figure 10.36 Demand for At-home Self Testing Ovulation Test Kits in Rest of North America, Till 2035 (Million Units)
- Figure 10.37 Demand for At-home Self Testing Ovulation Test Kits in Europe, Till 2035 (Million Units)
- Figure 10.38 Demand for At-home Self Testing Ovulation Test Kits in the UK, Till 2035 (Million Units)
- Figure 10.39 Demand for At-home Self Testing Ovulation Test Kits in Germany, Till 2035 (Million Units)
- Figure 10.40 Demand for At-home Self Testing Ovulation Test Kits in Spain, Till 2035 (Million Units)
- Figure 10.41 Demand for At-home Self Testing Ovulation Test Kits in Italy, Till 2035 (Million Units)
- Figure 10.42 Demand for At-home Self Testing Ovulation Test Kits in France, Till 2035 (Million Units)
- Figure 10.43 Demand for At-home Self Testing Ovulation Test Kits in Rest of Europe, Till 2035 (Million Units)
- Figure 10.44 Demand for At-home Self Testing Ovulation Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Figure 10.45 Demand for At-home Self Testing Ovulation Test Kits in India, Till 2035 (Million Units)
- Figure 10.46 Demand for At-home Self Testing Ovulation Test Kits in China, Till 2035 (Million Units)
- Figure 10.47 Demand for At-home Self Testing Ovulation Test Kits in Japan, Till 2035 (Million Units)
- Figure 10.48 Demand for At-home Self Testing Ovulation Test Kits in South Korea, Till 2035 (Million Units)
- Figure 10.49 Demand for At-home Self Testing Ovulation Test Kits in Indonesia, Till 2035 (Million Units)
- Figure 10.50 Demand for At-home Self Testing Ovulation Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Figure 10.51 Demand for At-home Self Testing Ovulation Test Kits in Africa, Till 2035 (Million Units)
- Figure 10.52 Demand for At-home Self Testing Ovulation Test Kits in Nigeria, Till 2035 (Million Units)
- Figure 10.53 Demand for At-home Self Testing Ovulation Test Kits in Ethiopia, Till 2035 (Million Units)
- Figure 10.54 Demand for At-home Self Testing Ovulation Test Kits in Egypt, Till 2035 (Million Units)
- Figure 10.55 Demand for At-home Self Testing Ovulation Test Kits in Rest of Africa, Till 2035 (Million Units)
- Figure 10.56 Demand for At-home Self Testing Ovulation Test Kits in South America, Till 2035 (Million Units)
- Figure 10.57 Demand for At-home Self Testing Ovulation Test Kits in Argentina, Till 2035 (Million Units)
- Figure 10.58 Demand for At-home Self Testing Ovulation Test Kits in Brazil, Till 2035 (Million Units)
- Figure 10.59 Demand for At-home Self Testing Ovulation Test Kits in Rest of South America, Till 2035 (Million Units)
- Figure 10.60 Demand for At-home Self Testing HIV Test Kits, Till 2035 (Million Units)
- Figure 10.61 Demand for At-home Self Testing HIV Test Kits in North America, Till 2035 (Million Units)
- Figure 10.62 Demand for At-home Self Testing HIV Test Kits in the US, Till 2035 (Million Units)
- Figure 10.63 Demand for At-home Self Testing HIV Test Kits in Canada, Till 2035 (Million Units)
- Figure 10.64 Demand for At-home Self Testing HIV Test Kits in Rest of North America, Till 2035 (Million Units)
- Figure 10.65 Demand for At-home Self Testing HIV Test Kits in Europe, Till 2035 (Million Units)
- Figure 10.66 Demand for At-home Self Testing HIV Test Kits in the UK, Till 2035 (Million Units)
- Figure 10.67 Demand for At-home Self Testing HIV Test Kits in Germany, Till 2035 (Million Units)
- Figure 10.68 Demand for At-home Self Testing HIV Test Kits in Spain, Till 2035 (Million Units)
- Figure 10.69 Demand for At-home Self Testing HIV Test Kits in Italy, Till 2035 (Million Units)
- Figure 10.70 Demand for At-home Self Testing HIV Test Kits in France, Till 2035 (Million Units)
- Figure 10.71 Demand for At-home Self Testing HIV Test Kits in Rest of Europe, Till 2035 (Million Units)
- Figure 10.72 Demand for At-home Self Testing HIV Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Figure 10.73 Demand for At-home Self Testing HIV Test Kits in India, Till 2035 (Million Units)
- Figure 10.74 Demand for At-home Self Testing HIV Test Kits in China, Till 2035 (Million Units)
- Figure 10.75 Demand for At-home Self Testing HIV Test Kits in Japan, Till 2035 (Million Units)
- Figure 10.76 Demand for At-home Self Testing HIV Test Kits in South Korea, Till 2035 (Million Units)
- Figure 10.77 Demand for At-home Self Testing HIV Test Kits in Indonesia, Till 2035 (Million Units)
- Figure 10.78 Demand for At-home Self Testing HIV Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Figure 10.79 Demand for At-home Self Testing HIV Test Kits in Africa, Till 2035 (Million Units)
- Figure 10.80 Demand for At-home Self Testing HIV Test Kits in Nigeria, Till 2035 (Million Units)
- Figure 10.81 Demand for At-home Self Testing HIV Test Kits in Ethiopia, Till 2035 (Million Units)
- Figure 10.82 Demand for At-home Self Testing HIV Test Kits in Egypt, Till 2035 (Million Units)
- Figure 10.83 Demand for At-home Self Testing HIV Test Kits in Rest of Africa, Till 2035 (Million Units)
- Figure 10.84 Demand for At-home Self Testing HIV Test Kits in South America, Till 2035 (Million Units)
- Figure 10.85 Demand for At-home Self Testing HIV Test Kits in Argentina, Till 2035 (Million Units)
- Figure 10.86 Demand for At-home Self Testing HIV Test Kits in Brazil, Till 2035 (Million Units)
- Figure 10.87 Demand for At-home Self Testing HIV Test Kits in Rest of South America, Till 2035 (Million Units)
- Figure 10.88 Demand for At-home Self-testing Diabetes Test Kits, Till 2035 (Million Units)
- Figure 10.89 Demand for At-home Self-testing Diabetes Test Kits in North America, Till 2035 (Million Units)
- Figure 10.90 Demand for At-home Self-testing Diabetes Test Kits in the US, Till 2035 (Million Units)
- Figure 10.91 Demand for At-home Self-testing Diabetes Test Kits in Canada, Till 2035 (Million Units)
- Figure 10.92 Demand for At-home Self-testing Diabetes Test Kits in Rest of North America, Till 2035 (Million Units)
- Figure 10.93 Demand for At-home Self-testing Diabetes Test Kits in Europe, Till 2035 (Million Units)
- Figure 10.94 Demand for At-home Self-testing Diabetes Test Kits in the UK, Till 2035 (Million Units)
- Figure 10.95 Demand for At-home Self-testing Diabetes Test Kits in Germany, Till 2035 (Million Units)
- Figure 10.96 Demand for At-home Self-testing Diabetes Test Kits in Spain, Till 2035 (Million Units)
- Figure 10.97 Demand for At-home Self-testing Diabetes Test Kits in Italy, Till 2035 (Million Units)
- Figure 10.98 Demand for At-home Self-testing Diabetes Test Kits in France, Till 2035 (Million Units)
- Figure 10.99 Demand for At-home Self-testing Diabetes Test Kits in Rest of Europe, Till 2035 (Million Units)
- Figure 10.100 Demand for At-home Self-testing Diabetes Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Figure 10.101 Demand for At-home Self-testing Diabetes Test Kits in India, Till 2035 (Million Units)
- Figure 10.102 Demand for At-home Self-testing Diabetes Test Kits in China, Till 2035 (Million Units)
- Figure 10.103 Demand for At-home Self-testing Diabetes Test Kits in Japan, Till 2035 (Million Units)
- Figure 10.104 Demand for At-home Self-testing Diabetes Test Kits in South Korea, Till 2035 (Million Units)
- Figure 10.105 Demand for At-home Self-testing Diabetes Test Kits in Indonesia, Till 2035 (Million Units)
- Figure 10.106 Demand for At-home Self-testing Diabetes Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Figure 10.107 Demand for At-home Self-testing Diabetes Test Kits in Africa, Till 2035 (Million Units)
- Figure 10.118 Demand for At-home Self-testing Diabetes Test Kits in Nigeria, Till 2035 (Million Units)
- Figure 10.109 Demand for At-home Self-testing Diabetes Test Kits in Ethiopia, Till 2035 (Million Units)
- Figure 10.110 Demand for At-home Self-testing Diabetes Test Kits in Egypt, Till 2035 (Million Units)
- Figure 10.111 Demand for At-home Self-testing Diabetes Test Kits in Rest of Africa, Till 2035 (Million Units)
- Figure 10.112 Demand for At-home Self-testing Diabetes Test Kits in South America, Till 2035 (Million Units)
- Figure 10.113 Demand for At-home Self-testing Diabetes Test Kits in Argentina, Till 2035 (Million Units)
- Figure 10.114 Demand for At-home Self-testing Diabetes Test Kits in Brazil, Till 2035 (Million Units)
- Figure 10.115 Demand for At-home Self-testing Diabetes Test Kits in Rest of South America, Till 2035 (Million Units)
- Figure 10.116 Demand for At-home Self-testing Colon Cancer Test Kits, Till 2035 (Million Units)
- Figure 10.117 Demand for At-home Self-testing Colon Cancer Test Kits in North America, Till 2035 (Million Units)
- Figure 10.118 Demand for At-home Self-testing Colon Cancer Test Kits in the US, Till 2035 (Million Units)
- Figure 10.119 Demand for At-home Self-testing Colon Cancer Test Kits in Canada, Till 2035 (Million Units)
- Figure 10.120 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of North America, Till 2035 (Million Units)
- Figure 10.121 Demand for At-home Self-testing Colon Cancer Test Kits in Europe, Till 2035 (Million Units)
- Figure 10.122 Demand for At-home Self-testing Colon Cancer Test Kits in the UK, Till 2035 (Million Units)
- Figure 10.123 Demand for At-home Self-testing Colon Cancer Test Kits in Germany, Till 2035 (Million Units)
- Figure 10.124 Demand for At-home Self-testing Colon Cancer Test Kits in Spain, Till 2035 (Million Units)
- Figure 10.125 Demand for At-home Self-testing Colon Cancer Test Kits in Italy, Till 2035 (Million Units)
- Figure 10.126 Demand for At-home Self-testing Colon Cancer Test Kits in France, Till 2035 (Million Units)
- Figure 10.127 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Europe, Till 2035 (Million Units)
- Figure 10.128 Demand for At-home Self-testing Colon Cancer Test Kits in Asia-Pacific, Till 2035 (Million Units)
- Figure 10.129 Demand for At-home Self-testing Colon Cancer Test Kits in India, Till 2035 (Million Units)
- Figure 10.130 Demand for At-home Self-testing Colon Cancer Test Kits in China, Till 2035 (Million Units)
- Figure 10.131 Demand for At-home Self-testing Colon Cancer Test Kits in Japan, Till 2035 (Million Units)
- Figure 10.132 Demand for At-home Self-testing Colon Cancer Test Kits in South Korea, Till 2035 (Million Units)
- Figure 10.133 Demand for At-home Self-testing Colon Cancer Test Kits in Indonesia, Till 2035 (Million Units)
- Figure 10.134 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Asia-Pacific, Till 2035 (Million Units)
- Figure 10.135 Demand for At-home Self-testing Colon Cancer Test Kits in Africa, Till 2035 (Million Units)
- Figure 10.136 Demand for At-home Self-testing Colon Cancer Test Kits in Nigeria, Till 2035 (Million Units)
- Figure 10.137 Demand for At-home Self-testing Colon Cancer Test Kits in Ethiopia, Till 2035 (Million Units)
- Figure 10.138 Demand for At-home Self-testing Colon Cancer Test Kits in Egypt, Till 2035 (Million Units)
- Figure 10.139 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of Africa, Till 2035 (Million Units)
- Figure 10.140 Demand for At-home Self-testing Colon Cancer Test Kits in South America, Till 2035 (Million Units)
- Figure 10.141 Demand for At-home Self-testing Colon Cancer Test Kits in Argentina, Till 2035 (Million Units)
- Figure 10.142 Demand for At-home Self-testing Colon Cancer Test Kits in Brazil, Till 2035 (Million Units)
- Figure 10.143 Demand for At-home Self-testing Colon Cancer Test Kits in Rest of South America, Till 2035 (Million Units)
- Figure 11.1 Global At-home Self-testing Kits Market, Till 2035 (USD Billion)
- Figure 11.2 At-home Self-testing Kits Market: Distribution by Geography, Till 2035 (USD Billion)
- Figure 11.3 At-home Self-testing Kits Market: Distribution by Target Therapeutic Area, Till 2035 (USD Billion)
- Figure 11.4 At-home Self-testing Kits Market for Pregnancy Detection, Till 2035 (USD Billion)
- Figure 11.5 At-home Self-testing Kits Market for Pregnancy Detection in North America, Till 2035 (USD Billion)
- Figure 11.6 At-home Self-testing Kits Market for Pregnancy Detection in the US, Till 2035 (USD Billion)
- Figure 11.7 At-home Self-testing Kits Market for Pregnancy Detection in Canada, Till 2035 (USD Billion)
- Figure 11.8 At-home Self-testing Kits Market for Pregnancy Detection in Rest of North America, Till 2035 (USD Billion)
- Figure 11.9 At-home Self-testing Kits Market for Pregnancy Detection in Europe, Till 2035 (USD Billion)
- Figure 11.10 At-home Self-testing Kits Market for Pregnancy Detection in the UK, Till 2035 (USD Billion)
- Figure 11.11 At-home Self-testing Kits Market for Pregnancy Detection in Germany, Till 2035 (USD Billion)
- Figure 11.12 At-home Self-testing Kits Market for Pregnancy Detection in Spain, Till 2035 (USD Billion)
- Figure 11.13 At-home Self-testing Kits Market for Pregnancy Detection in Italy, Till 2035 (USD Billion)
- Figure 11.14 At-home Self-testing Kits Market for Pregnancy Detection in France, Till 2035 (USD Billion)
- Figure 11.15 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Europe, Till 2035 (USD Billion)
- Figure 11.16 At-home Self-testing Kits Market for Pregnancy Detection in Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.17 At-home Self-testing Kits Market for Pregnancy Detection in India, Till 2035 (USD Billion)
- Figure 11.18 At-home Self-testing Kits Market for Pregnancy Detection in China, Till 2035 (USD Billion)
- Figure 11.19 At-home Self-testing Kits Market for Pregnancy Detection in Japan, Till 2035 (USD Billion)
- Figure 11.20 At-home Self-testing Kits Market for Pregnancy Detection in South Korea, Till 2035 (USD Billion)
- Figure 11.21 At-home Self-testing Kits Market for Pregnancy Detection in Indonesia, Till 2035 (USD Billion)
- Figure 11.22 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.23 At-home Self-testing Kits Market for Pregnancy Detection in Africa, Till 2035 (USD Billion)
- Figure 11.24 At-home Self-testing Kits Market for Pregnancy Detection in Nigeria, Till 2035 (USD Billion)
- Figure 11.25 At-home Self-testing Kits Market for Pregnancy Detection in Ethiopia, Till 2035 (USD Billion)
- Figure 11.26 At-home Self-testing Kits Market for Pregnancy Detection in Egypt, Till 2035 (USD Billion)
- Figure 11.27 At-home Self-testing Kits Market for Pregnancy Detection in Rest of Africa, Till 2035 (USD Billion)
- Figure 11.28 At-home Self-testing Kits Market for Pregnancy Detection in South America, Till 2035 (USD Billion)
- Figure 11.29 At-home Self-testing Kits Market for Pregnancy Detection in Argentina, Till 2035 (USD Billion)
- Figure 11.30 At-home Self-testing Kits Market for Pregnancy Detection in Brazil, Till 2035 (USD Billion)
- Figure 11.31 At-home Self-testing Kits Market for Pregnancy Detection in Rest of South America, Till 2035 (USD Billion)
- Figure 11.32 At-home Self-testing Kits Market for Ovulation Monitoring, Till 2035 (USD Billion)
- Figure 11.33 At-home Self-testing Kits Market for Ovulation Monitoring in North America, Till 2035 (USD Billion)
- Figure 11.34 At-home Self-testing Kits Market for Ovulation Monitoring in the US, Till 2035 (USD Billion)
- Figure 11.35 At-home Self-testing Kits Market for Ovulation Monitoring in Canada, Till 2035 (USD Billion)
- Figure 11.36 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of North America, Till 2035 (USD Billion)
- Figure 11.37 At-home Self-testing Kits Market for Ovulation Monitoring in Europe, Till 2035 (USD Billion)
- Figure 11.38 At-home Self-testing Kits Market for Ovulation Monitoring in the UK, Till 2035 (USD Billion)
- Figure 11.39 At-home Self-testing Kits Market for Ovulation Monitoring in Germany, Till 2035 (USD Billion)
- Figure 11.40 At-home Self-testing Kits Market for Ovulation Monitoring in Spain, Till 2035 (USD Billion)
- Figure 11.41 At-home Self-testing Kits Market for Ovulation Monitoring in Italy, Till 2035 (USD Billion)
- Figure 11.42 At-home Self-testing Kits Market for Ovulation Monitoring in France, Till 2035 (USD Billion)
- Figure 11.43 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Europe, Till 2035 (USD Billion)
- Figure 11.44 At-home Self-testing Kits Market for Ovulation Monitoring in Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.45 At-home Self-testing Kits Market for Ovulation Monitoring in India, Till 2035 (USD Billion)
- Figure 11.46 At-home Self-testing Kits Market for Ovulation Monitoring in China, Till 2035 (USD Billion)
- Figure 11.47 At-home Self-testing Kits Market for Ovulation Monitoring in Japan, Till 2035 (USD Billion)
- Figure 11.48 At-home Self-testing Kits Market for Ovulation Monitoring in South Korea, Till 2035 (USD Billion)
- Figure 11.49 At-home Self-testing Kits Market for Ovulation Monitoring in Indonesia, Till 2035 (USD Billion)
- Figure 11.50 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.51 At-home Self-testing Kits Market for Ovulation Monitoring in Africa, Till 2035 (USD Billion)
- Figure 11.52 At-home Self-testing Kits Market for Ovulation Monitoring in Nigeria, Till 2035 (USD Billion)
- Figure 11.53 At-home Self-testing Kits Market for Ovulation Monitoring in Ethiopia, Till 2035 (USD Billion)
- Figure 11.54 At-home Self-testing Kits Market for Ovulation Monitoring in Egypt, Till 2035 (USD Billion)
- Figure 11.55 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of Africa, Till 2035 (USD Billion)
- Figure 11.56 At-home Self-testing Kits Market for Ovulation Monitoring in South America, Till 2035 (USD Billion)
- Figure 11.57 At-home Self-testing Kits Market for Ovulation Monitoring in Argentina, Till 2035 (USD Billion)
- Figure 11.58 At-home Self-testing Kits Market for Ovulation Monitoring in Brazil, Till 2035 (USD Billion)
- Figure 11.59 At-home Self-testing Kits Market for Ovulation Monitoring in Rest of South America, Till 2035 (USD Billion)
- Figure 11.60 At-home Self-testing Kits Market for HIV Detection, Till 2035 (USD Billion)
- Figure 11.61 At-home Self-testing Kits Market for HIV Detection in North America, Till 2035 (USD Billion)
- Figure 11.62 At-home Self-testing Kits Market for HIV Detection in the US, Till 2035 (USD Billion)
- Figure 11.63 At-home Self-testing Kits Market for HIV Detection in Canada, Till 2035 (USD Billion)
- Figure 11.64 At-home Self-testing Kits Market for HIV Detection in Rest of North America, Till 2035 (USD Billion)
- Figure 11.65 At-home Self-testing Kits Market for HIV Detection in Europe, Till 2035 (USD Billion)
- Figure 11.66 At-home Self-testing Kits Market for HIV Detection in the UK, Till 2035 (USD Billion)
- Figure 11.67 At-home Self-testing Kits Market for HIV Detection in Germany, Till 2035 (USD Billion)
- Figure 11.68 At-home Self-testing Kits Market for HIV Detection in Spain, Till 2035 (USD Billion)
- Figure 11.69 At-home Self-testing Kits Market for HIV Detection in Italy, Till 2035 (USD Billion)
- Figure 11.70 At-home Self-testing Kits Market for HIV Detection in France, Till 2035 (USD Billion)
- Figure 11.71 At-home Self-testing Kits Market for HIV Detection in Rest of Europe, Till 2035 (USD Billion)
- Figure 11.72 At-home Self-testing Kits Market for HIV Detection in Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.73 At-home Self-testing Kits Market for HIV Detection in India, Till 2035 (USD Billion)
- Figure 11.74 At-home Self-testing Kits Market for HIV Detection in China, Till 2035 (USD Billion)
- Figure 11.75 At-home Self-testing Kits Market for HIV Detection in Japan, Till 2035 (USD Billion)
- Figure 11.76 At-home Self-testing Kits Market for HIV Detection in South Korea, Till 2035 (USD Billion)
- Figure 11.77 At-home Self-testing Kits Market for HIV Detection in Indonesia, Till 2035 (USD Billion)
- Figure 11.78 At-home Self-testing Kits Market for HIV Detection in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.79 At-home Self-testing Kits Market for HIV Detection in Africa, Till 2035 (USD Billion)
- Figure 11.80 At-home Self-testing Kits Market for HIV Detection in Nigeria, Till 2035 (USD Billion)
- Figure 11.81 At-home Self-testing Kits Market for HIV Detection in Ethiopia, Till 2035 (USD Billion)
- Figure 11.82 At-home Self-testing Kits Market for HIV Detection in Egypt, Till 2035 (USD Billion)
- Figure 11.83 At-home Self-testing Kits Market for HIV Detection in Rest of Africa, Till 2035 (USD Billion)
- Figure 11.84 At-home Self-testing Kits Market for HIV Detection in South America, Till 2035 (USD Billion)
- Figure 11.85 At-home Self-testing Kits Market for HIV Detection in Argentina, Till 2035 (USD Billion)
- Figure 11.86 At-home Self-testing Kits Market for HIV Detection in Brazil, Till 2035 (USD Billion)
- Figure 11.87 At-home Self-testing Kits Market for HIV Detection in Rest of South America, Till 2035 (USD Billion)
- Figure 11.88 At-home Self-testing Kits Market for Diabetes Monitoring, Till 2035 (USD Billion)
- Figure 11.89 At-home Self-testing Kits Market for Diabetes Monitoring in North America, Till 2035 (USD Billion)
- Figure 11.90 At-home Self-testing Kits Market for Diabetes Monitoring in the US, Till 2035 (USD Billion)
- Figure 11.91 At-home Self-testing Kits Market for Diabetes Monitoring in Canada, Till 2035 (USD Billion)
- Figure 11.92 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of North America, Till 2035 (USD Billion)
- Figure 11.93 At-home Self-testing Kits Market for Diabetes Monitoring in Europe, Till 2035 (USD Billion)
- Figure 11.94 At-home Self-testing Kits Market for Diabetes Monitoring in the UK, Till 2035 (USD Billion)
- Figure 11.95 At-home Self-testing Kits Market for Diabetes Monitoring in Germany, Till 2035 (USD Billion)
- Figure 11.96 At-home Self-testing Kits Market for Diabetes Monitoring in Spain, Till 2035 (USD Billion)
- Figure 11.97 At-home Self-testing Kits Market for Diabetes Monitoring in Italy, Till 2035 (USD Billion)
- Figure 11.98 At-home Self-testing Kits Market for Diabetes Monitoring in France, Till 2035 (USD Billion)
- Figure 11.99 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Europe, Till 2035 (USD Billion)
- Figure 11.100 At-home Self-testing Kits Market for Diabetes Monitoring in Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.101 At-home Self-testing Kits Market for Diabetes Monitoring in India, Till 2035 (USD Billion)
- Figure 11.102 At-home Self-testing Kits Market for Diabetes Monitoring in China, Till 2035 (USD Billion)
- Figure 11.103 At-home Self-testing Kits Market for Diabetes Monitoring in Japan, Till 2035 (USD Billion)
- Figure 11.104 At-home Self-testing Kits Market for Diabetes Monitoring in South Korea, Till 2035 (USD Billion)
- Figure 11.105 At-home Self-testing Kits Market for Diabetes Monitoring in Indonesia, Till 2035 (USD Billion)
- Figure 11.106 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.107 At-home Self-testing Kits Market for Diabetes Monitoring in Africa, Till 2035 (USD Billion)
- Figure 11.108 At-home Self-testing Kits Market for Diabetes Monitoring in Nigeria, Till 2035 (USD Billion)
- Figure 11.109 At-home Self-testing Kits Market for Diabetes Monitoring in Ethiopia, Till 2035 (USD Billion)
- Figure 11.110 At-home Self-testing Kits Market for Diabetes Monitoring in Egypt, Till 2035 (USD Billion)
- Figure 11.111 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of Africa, Till 2035 (USD Billion)
- Figure 11.112 At-home Self-testing Kits Market for Diabetes Monitoring in South America, Till 2035 (USD Billion)
- Figure 11.113 At-home Self-testing Kits Market for Diabetes Monitoring in Argentina, Till 2035 (USD Billion)
- Figure 11.114 At-home Self-testing Kits Market for Diabetes Monitoring in Brazil, Till 2035 (USD Billion)
- Figure 11.115 At-home Self-testing Kits Market for Diabetes Monitoring in Rest of South America, Till 2035 (USD Billion)
- Figure 11.116 At-home Self-testing Kits Market for Colon Cancer Detection, Till 2035 (USD Billion)
- Figure 11.117 At-home Self-testing Kits Market for Colon Cancer Detection in North America, Till 2035 (USD Billion)
- Figure 11.118 At-home Self-testing Kits Market for Colon Cancer Detection in the US, Till 2035 (USD Billion)
- Figure 11.119 At-home Self-testing Kits Market for Colon Cancer Detection in Canada, Till 2035 (USD Billion)
- Figure 11.120 At-home Self-testing Kits Market for Colon Cancer Detection in Rest of North America, Till 2035 (USD Billion)
- Figure 11.121 At-home Self-testing Kits Market for Colon Cancer Detection in Europe, Till 2035 (USD Billion)
- Figure 11.122 At-home Self-testing Kits Market for Colon Cancer Detection in the UK, Till 2035 (USD Billion)
- Figure 11.123 At-home Self-testing Kits Market for Colon Cancer Detection in Germany, Till 2035 (USD Billion)
- Figure 11.124 At-home Self-testing Kits Market for Colon Cancer Detection in Spain, Till 2035 (USD Billion)
- Figure 11.125 At-home Self-testing Kits Market for Colon Cancer Detection in Italy, Till 2035 (USD Billion)
- Figure 11.126 At-home Self-testing Kits Market for Colon Cancer Detection in France, Till 2035 (USD Billion)
- Figure 11.127 At-home Self-testing Kits Market for Colon Cancer Detection in Rest of Europe, Till 2035 (USD Billion)
- Figure 11.128 At-home Self-testing Kits Market for Colon Cancer Detection in Asia-Pacific, Till 2035 (USD Billion)
- Figure 11.129 At-home Self-testing Kits Market for Colon Cancer De




