PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1687734

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1687734
Global Xerostomia (Dry Mouth Disease) Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
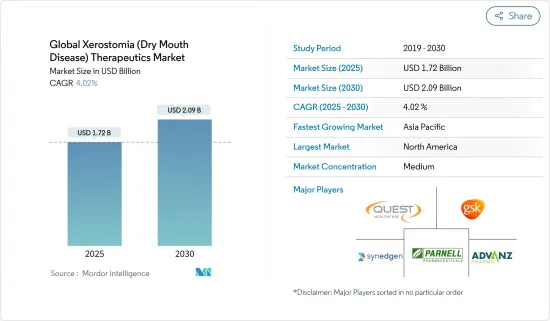
The Global Xerostomia Therapeutics Market size is estimated at USD 1.72 billion in 2025, and is expected to reach USD 2.09 billion by 2030, at a CAGR of 4.02% during the forecast period (2025-2030).
Xerostomia, commonly referred to as dry mouth, is a condition characterized by the reduced production of saliva. It can be caused by various factors, including medications, certain medical conditions, and radiation therapy. The dry mouth condition can lead to difficulties in speaking, swallowing, increased risk of dental problems, and overall discomfort. The therapeutics market for xerostomia includes several product categories such as salivary stimulants, saliva substitutes, and other agents designed to alleviate symptoms.
The growth of the market is mainly driven by the increasing prevalence of conditions that contribute to xerostomia, such as diabetes, autoimmune diseases (like Sjogren's syndrome), and the side effects of certain medications (particularly antidepressants, antihistamines, and cancer therapies) has heightened the demand for effective treatment solutions. Additionally, an aging population is contributing to a rise in xerostomia cases, as older adults are more likely to experience related health issues. For instance, according to the data published by the Institute for Health Metrics and Evaluation in June 2023, more than half a billion people are living with diabetes worldwide, affecting men, women, and children of all ages in every country, and that number is projected to more than double to 1.3 billion people by 2043. Hyperglycemia, a common condition in diabetes, can result in dehydration and reduced saliva production due to osmotic diuresis. These complications contribute to a significant demand for therapeutics aimed at managing xerostomia in diabetic patients. Thus, the increasing prevalence of diabetes worldwide drives the demand for xerostomia therapeutics, thereby influencing market growth.
Additionally, increasing advancements in pharmaceutical research have led to the development of novel products, including saliva substitutes and stimulants, that offer improved efficacy and patient compliance which in turn is anticipated to boost market growth over the forecast period. For instance, according to an article published by Franziska Beier, Dental Tribune International in December 2023, researchers at the University of Leeds United Kingdom developed a saliva substitute to alleviate the discomfort of patients suffering from dry mouth. The novel solution mimics natural saliva in its ability to moisten the mouth and serve as a lubricant during food intake. It comes in a dairy and a vegan formulation, and in vitro experiments have found it to be more effective than other commercially available products. Such advancements in products lead to drive its adoption to treat drug mouth, thereby influencing market growth.
Furthermore, increasing investment in healthcare infrastructure and rising disposable incomes in emerging markets are enhancing access to these therapeutics, further fueling market growth.
Therefore, owing to the above-mentioned factors, such as increasing prevalence of conditions that contribute to xerostomia, such as diabetes and increasing advancements in pharmaceutical research have led to the development of novel products, the market studied is expected to grow over the forecast period. However, factors such as lack of awareness and unavailability of effective treatment will likely hinder the usage of xerostomia therapeutics products and can act as a restraining factor for the xerostomia therapeutics market.
Global Xerostomia (Dry Mouth Disease) Therapeutics Market Trends
Artificial Saliva/Saliva Substitutes Segment is Expected to Dominate the Market Over the Forecast Period
Reduced saliva production can cause tooth decay, mouth infections, changes in taste or smell, difficulty swallowing, pain, and other problems. Artificial saliva, available as a liquid or spray, is used to moisten the mouth and ease the discomfort of chronic dry mouth, especially with regular use. Many artificial saliva sprays and gels claim to work for up to two hours, but users often need more frequent applications, affecting sleep and daily activities. Additionally, several saliva sprays and gels have strong and unpleasant flavours. However, the recent use of naturally sweet xylitol has led to better-tasting alternatives.
Factors such as the growing burden of dry mouth disease and increasing research studies propel the segment's growth. For instance, according to the data published by the American Dental Association in April 2023, xerostomia affected 10% to 26% of men and 10% to 33% of women in 2023. Given the significant prevalence of xerostomia, there's a heightened demand for therapeutic treatments, propelling the growth of the market.
Numerous studies have shown the efficacy of several artificial saliva formulations for treating people affected by xerostomia. For instance, a March 2024 study in the Journal of Oral Medicine, Oral Surgery, Oral Pathology, and Oral Radiology assessed and compared the efficacy of two artificial saliva formulations: the sodium carboxymethylcellulose (SCMC) spray and the SCMC spray enhanced with B-glucan. Both the control and the B-glucan enriched artificial saliva showed improvement in the clinical signs and symptoms of xerostomia.
Additionally, a May 2024 article in BMC Oral Health highlighted that an artificial saliva spray could significantly alleviate dry mouth symptoms in COVID-19 patients undergoing non-invasive mechanical ventilation. Such clinical trials provide valuable insights into the safety and effectiveness of artificial saliva products, leading to greater confidence among healthcare providers and patients, thereby driving market growth. Hence, the segment is expected to register steady growth during the forecast period due to the factors above.
North America is Expected to Dominate the Xerostomia (Dry Mouth Disease) Therapeutics Market
North America is poised to lead the market, driven by increasing prevalence of conditions that contribute to xerostomia, such as Sjogren's syndrome, diabetes, and cancer treatments. Additionally, aging population, heightened awareness of xerostomia among healthcare professionals and patients, advancements in therapeutic options, including innovative saliva substitutes, stimulants, and moisture-retaining agents, and significant investment in research and development by pharmaceutical companies are further anticipated to drive segmental growth over the forecast period.
The increasing prevalence of diabetes is anticipated to boost market growth in North America, reflecting the need for effective management solutions to improve quality of life for those affected by diabetes. For instance, according to the data published by the Canadian Diabetes Association 2023, 4.1 million diabetic (type 1 and type 2 diagnosed) patients were recorded in 2023 in Canada and is projected to grow to around 5.2 million by 2033. Diabetes can cause damage to the salivary glands, leading to reduced saliva production and an increased risk of infections, cavities, and gum disease. Thus, the increasing prevalence of diabetes in Canada, along with its associated oral health complications, has created a growing demand for therapeutics aimed at managing xerostomia, thereby influencing market growth in the region.
Furthermore, the growing number of investments by various institutes, including pharmaceutical companies, research organizations, and venture capitalists, is significantly driving the xerostomia (dry mouth disease) therapeutics market in North America. For instance, in October 2024, an associate professor of oral biology at the University at Buffalo's School of Dental Medicine, United States secured a five-year grant renewal worth USD 2.2 million from the National Institute of Dental and Craniofacial Research (NIDCR), part of the National Institutes of Health. This funding aims to alleviate the suffering of patients with conditions like Sjogren's disease, which depletes moisture from various body glands. Such funding enables the development and commercialization of innovative treatment solutions aimed at alleviating symptoms associated with xerostomia, thereby expanding the market's potential and improving patient outcomes in this often-overlooked condition.
Moreover, strategic initiatives by key players such as product approvals and the introduction of innovative therapies for xerostomia (dry mouth disease), enhance treatment options and drive market growth by addressing unmet patient needs and improving quality of life. For instance, in October 2024, RiboX Therapeutics, United States received Food and Drug Administration (FDA) clearance for the investigational new drug (IND) application of RXRG001, introducing the first circular RNA therapy designed to treat radiation-induced xerostomia and hyposalivation. Such product approvals not only enhances clinical outcomes for patients but also stimulates market growth by encouraging further research and investment in xerostomia therapeutics, thereby boosting market growth,
In summary, the increasing prevalence of diabetes, the growing number of investments by various institutions, and product approvals are likely to increase the demand for xerostomia (dry mouth disease) therapeutics in North America and drive the market's growth during the forecast period.
Global Xerostomia (Dry Mouth Disease) Therapeutics Industry Overview
The xerostomia (dry mouth disease) therapeutics market is moderately competitive. Key players in the xerostomia therapeutics market are focusing on developing their product portfolio, acquiring emerging players, and distributing agreements to increase their geographical presence. Some of the key players dominating the xerostomia therapeutics market are Quest Healthcare, GlaxoSmithKline plc, Pharmascience Inc., ADVANZ PHARMA, Lupin, Parnell Pharmaceuticals Inc., Sun Pharmaceutical Industries Ltd., and Synedgen, Inc., among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Incidences of Xerostomia in the Aging Population
- 4.2.2 Growing Awareness about Xerostomia-related Products
- 4.2.3 Development of Innovative Therapeutic Products for Xerostomia
- 4.3 Market Restraints
- 4.3.1 High Cost of Medications Associated with Xerostomia Therapies
- 4.3.2 Strict Regulations Pertaining to Selling Xerostomia-related Products
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Type
- 5.1.1 Artificial Saliva/Saliva Substitutes
- 5.1.2 Salivary Stimulants
- 5.2 By Product
- 5.2.1 Drugs
- 5.2.2 Salivary Pens
- 5.2.3 Other Products
- 5.3 By Distribution Channel
- 5.3.1 Hospital Pharmacy
- 5.3.2 Retail Pharmacy
- 5.3.3 Online Pharmacy
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Quest Healthcare
- 6.1.2 Gilde Healthcare
- 6.1.3 GlaxoSmithKline plc
- 6.1.4 Parnell Pharmaceuticals, Inc.
- 6.1.5 Lupin
- 6.1.6 Pharmascience Inc.
- 6.1.7 OraCoat
- 6.1.8 Sun Pharmaceutical Industries Ltd
- 6.1.9 Synedgen, Inc.
- 6.1.10 ADVANZ PHARMA
- 6.1.11 Saliwell Ltd
- 6.1.12 3M
- 6.1.13 Pendopharm
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




