PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1685898

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1685898
Preventive Vaccines - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
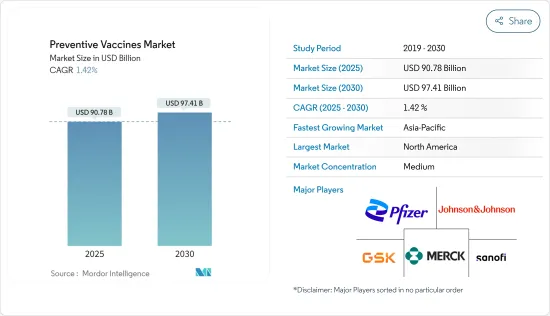
The Preventive Vaccines Market size is estimated at USD 90.78 billion in 2025, and is expected to reach USD 97.41 billion by 2030, at a CAGR of 1.42% during the forecast period (2025-2030).
Factors such as the growing prevalence of infectious diseases, innovative technology in vaccine development, increased funding from government and international organizations, and the increasing government focus on immunization programs are boosting the market's growth.
The rising incidences of infectious diseases are the key factor expected to drive the demand for preventive vaccines over the forecast period. For instance, according to the World Health Organization data published in November 2023, tuberculosis was the second most deadly infectious disease globally, following COVID-19. In 2022, an estimated 10.6 million individuals globally contracted tuberculosis (TB), comprising 5.8 million men, 3.5 million women, and 1.3 million children. Additionally, per the Centers for Disease Control and Prevention (CDC), the US TB rate climbed by 15% in March 2024, from 2.5 per 100,000 individuals in 2022 to 2.9 in 2023.
Thus, the rising burden of tuberculosis among the population increases the need for its prevention, which, in turn, is anticipated to propel the market's growth over the forecast period. Additionally, according to data published by the World Health Organization (WHO) in July 2023, globally, an estimated 58 million people had chronic hepatitis C virus, and about 1.5 million new infections occur annually. According to the same source, the hepatitis C virus is a blood-borne pathogen, and the most common form of the disease is exposure to small amounts of blood. Thus, the increasing burden of hepatitis is propelling the growth of the market.
The rapid advancements in vaccine technology are fueled by the introduction of genetic engineering, vaccine-delivering technology, and proteomics. For instance, in January 2024, the National Medical Products Administration of China granted regulatory approval for CSPC Pharmaceutical Group's candidate vaccine for the respiratory syncytial virus (RSV) to commence human clinical trials. This is expected to increase the development of preventive vaccine products, bolstering the market's growth.
Rising government initiatives in organizing immunization programs to prevent the spread of infectious diseases and the need for vaccination are expected to increase the market's growth over the forecast period. For instance, in February 2024, the Government of India initiated a vaccination program targeting girls aged 9 to 14 aimed at preventing cervical cancer. Furthermore, the government is actively promoting this vaccination among the eligible age group. Therefore, owing to these factors, the market is expected to grow over the forecast period. However, the risk of adverse effects and the high cost of vaccine development will likely impede the market's growth over the forecast period.
Preventive Vaccines Market Trends
The Hepatitis Segment is Expected to Witness Significant Growth Over the Forecast Period
Hepatitis, characterized by liver inflammation, can arise from various sources, including viruses, alcohol, and drugs. The predominant strains are hepatitis A, B, and C, each stemming from distinct viruses. Common symptoms encompass jaundice, fatigue, and abdominal discomfort. Treatment strategies hinge on the underlying cause and the condition's severity.
The rise in hepatitis cases is likely to drive the growth of the preventive vaccines market due to increasing demand for effective vaccination solutions. As hepatitis remains a significant global health issue, especially in areas with high infection rates, both public health initiatives and individual awareness are leading to more substantial investment in vaccine development and distribution. For instance, according to the World Health Organization data updated in 2024, in 2022, 304 million individuals globally were diagnosed with chronic viral hepatitis B and C. Further, 2.2 million new infections of these chronic viral hepatitis strains occurred in 2022. Therefore, as the demand for effective vaccination solutions increases to combat the rising number of hepatitis cases, there is a stronger emphasis on developing and distributing vaccines. This heightened focus on prevention and public health drives investment and innovation in the segment.
Government initiatives play a crucial role in driving the preventive vaccines market by promoting vaccination programs, funding research, and implementing public health policies. Governments often launch vaccination campaigns, provide subsidies, and establish immunization guidelines to enhance public access to vaccines. For instance, in June 2024, Gavi unveiled new vaccination initiatives targeting preventive measures against Ebola, routine multivalent meningitis, human rabies, and the hepatitis B birth dose. As per the same source, Gavi is extending its support to lower-income nations, facilitating the routine administration of the human rabies vaccine for post-exposure prophylaxis alongside the multivalent meningococcal conjugate and hepatitis B birth dose vaccines.
Therefore, the segment is expected to grow over the forecast period due to factors such as the rising burden of hepatitis A, B, and C and increasing government initiatives to boost the preventive vaccines for hepatitis.
North America is Expected to Hold a Significant Market Share Over the Forecast Period
The preventive vaccines market in North America is expected to grow over the forecast period owing to factors such as the rising prevalence of infectious diseases, increasing demand for vaccines, growing geriatric population prone to develop infectious diseases, and the presence of key market players.
The rising burden of infectious diseases, such as COVID-19, drives the preventive vaccines market by significantly increasing vaccine demand. The urgent need to control widespread outbreaks accelerates vaccine development and broadens the range of available vaccines. This heightened demand leads to more significant investment in research, improved vaccine distribution, and the implementation of vaccination mandates. For instance, according to data published by the World Health Organization (WHO) in August 2024, about 7.6 million confirmed cases of COVID-19 were reported in Mexico. In addition, 222.23 million vaccine doses had been administered in the country by August 2024.
According to the same source, 711.81 million vaccine doses had been administered in the country by August 2024 in the United States. Therefore, the outbreak of infectious diseases like COVID-19 stimulates the preventive vaccines market by boosting demand, accelerating development, and expanding vaccine availability, which is expected to fuel the market's growth over the forecast period.
Increasing government focus on organizing immunization programs also contributes to the market's growth. For instance, in December 2023, Ontarians and other Canadians accessed free influenza vaccines under the Universal Influenza Immunization Program (UIIP). Ontario residents aged six months and older who work or attend school qualified for the influenza vaccine. Additionally, in February 2023, the Canadian government's immunization program advised that children should receive the polio vaccine at 2, 4, and 18 months, followed by a booster dose between the ages of 4 and 6. An extra dose of IPV can be given at six months, alongside DTap and Hib, for convenience. Therefore, government-led immunization programs drive the preventive vaccines market by expanding vaccine access, increasing public participation, and fostering investment in vaccine development, which will drive market growth in North America.
Growing funding from government and international organizations to accelerate the development of preventive vaccines in the country is also expected to boost the market's growth. For instance, according to the National Institute of Health, in March 2024, the US government allocated USD 364 million to combat hepatitis in 2023, following a USD 359 million investment in 2022. Therefore, increasing government funding for hepatitis propels the preventive vaccines market by supporting research, enhancing production capabilities, and expanding vaccination programs. Such investments foster broader vaccine availability and accessibility, ultimately strengthening global efforts to control and prevent hepatitis.
The rising focus of companies on adopting various business strategies, such as collaboration, expansion, and launches, is expected to create opportunities for the availability of preventive vaccines in the market. This is anticipated to fuel the market's growth over the forecast period. For instance, in October 2023, Pfizer received US Food and Drug Administration (FDA) approval for PENBRAYA, making it the first and only vaccine against the five most common serogroups of meningococcal disease in adolescents and young adults.
In addition, in August 2023, Pfizer received US FDA approval for ABRYSVO. This approval was for a specific use case: immunization of pregnant women between 32 and 36 weeks of gestation to protect their newborns from respiratory syncytial virus (RSV). It was the first and only US approval for a maternal vaccine to help protect infants at birth through six months of life from lower respiratory tract disease (LRTD) and severe LRTD due to RSV.
Therefore, the market is expected to grow over the forecast period due to factors such as the high incidences of infectious diseases, increasing immunization programs and campaigns, growing government funding for developing preventive vaccines, and increasing company activities.
Preventive Vaccines Industry Overview
The preventive vaccines market is fragmented and consists of several major players. A few of the major players currently dominating the market in terms of market share include GSK PLC, Johnson & Johnson Services Inc., Merck & Co., Pfizer Inc., and Sanofi.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Prevalence of Infectious Diseases
- 4.2.2 Innovative Technology in Vaccine Development
- 4.2.3 Increased Funding from Government and International Organizations
- 4.2.4 Increasing Government Focus on Immunization Programs
- 4.3 Market Restraints
- 4.3.1 Risk of Adverse Effects
- 4.3.2 High Cost of Vaccine Development
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Vaccine Type
- 5.1.1 Live/Attenuated Vaccines
- 5.1.2 Inactivated Vaccines
- 5.1.3 Subunit Vaccines
- 5.1.4 Toxoid Vaccines
- 5.1.5 mRNA Vaccines
- 5.1.6 Other Vaccine Types
- 5.2 By Disease Type
- 5.2.1 Pneumococcal
- 5.2.2 Poliovirus
- 5.2.3 Hepatitis
- 5.2.4 Influenza
- 5.2.5 Measles, Mumps, and Rubella (MMR)
- 5.2.6 COVID-19
- 5.2.7 Other Disease Types
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 AstraZeneca PLC
- 6.1.2 Emergent BioSolutions Inc.
- 6.1.3 Daiichi Sankyo Company Limited
- 6.1.4 GSK plc
- 6.1.5 Johnson & Johnson Services, Inc.
- 6.1.6 Merck & Co.
- 6.1.7 Novavax Inc.
- 6.1.8 Pfizer Inc.
- 6.1.9 Sanofi
- 6.1.10 Takeda Pharmaceutical Co. Ltd
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




