PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1521875

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1521875
Smart Inhalers - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
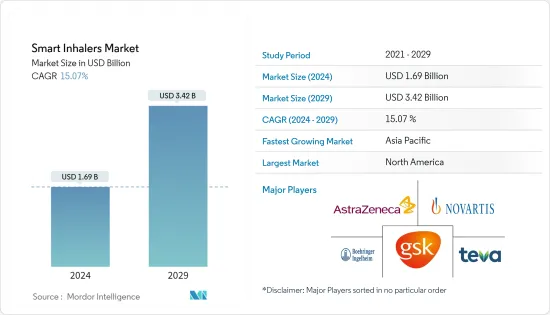
The Smart Inhalers Market size is estimated at USD 1.69 billion in 2024, and is expected to reach USD 3.42 billion by 2029, growing at a CAGR of 15.07% during the forecast period (2024-2029).
The pandemic accelerated the adoption of telehealth and remote patient monitoring. The surge in telemedicine during the pandemic amplified the significance of smart inhalers, facilitating remote patient monitoring and data collection. The increased demand for virtual healthcare services drove the adoption of remote solutions, aligning with the evolving landscape of healthcare delivery. With the increased focus on respiratory health during the pandemic, patients and healthcare providers became more conscious of the importance of adherence to inhaler regimens. Smart inhalers with reminder features helped patients stay on track. Smart inhalers helped reduce the burden on healthcare systems by enabling efficient monitoring and management of respiratory conditions remotely, freeing up resources to combat the pandemic. The overall effect was likely an increased awareness and adoption of smart inhaler technologies, especially for the management of respiratory conditions.
Therefore, the pandemic turned out to be a leading factor in the growth of the smart inhalers market. The industry is poised for significant growth, driven by an escalating prevalence of respiratory conditions, a growing emphasis on proactive and personalized healthcare, advancements in sensor technologies, and the increasing integration of digital health solutions into mainstream medical practices.
Furthermore, increasing air pollution, smoking, and the aging population contribute to a growing number of individuals affected by chronic respiratory disorders. A recent estimation based on data from 194 countries, published in the Global Initiative for Asthma Reports, 2020, concluded that each year, 4.0 million new cases of pediatric asthma might be attributable to NO2 pollution, accounting for 13% of global incidence. This contribution exceeded 20% of new asthma cases. The European analysis subset reported in the same paper estimated that 17% of the burden in Western Europe, 14% in Central Europe, and 17% in Eastern Europe was attributable to NO2. Patients with these diseases require effective management of their conditions to control symptoms and prevent exacerbations. Inhalers are a primary means of treatment for these conditions.
In addition, poor medication adherence is a significant issue in the management of chronic respiratory disorders. According to a study published by the National Library of Medicine in 2022, poor medication adherence and incorrect inhaler use lead to suboptimal asthma control. Medication adherence varies from 13% to 52% across the world. Several factors, such as illness perceptions, medication beliefs, forgetfulness, difficulty understanding inhaler techniques, attitude toward the illness, and self-efficacy, contribute to poor medication adherence. Many patients struggle to adhere to prescribed inhaler regimens, leading to suboptimal disease control. Smart inhalers offer features like reminders, dose tracking, and data sharing with healthcare providers. These features have been proven to significantly improve patient adherence to inhaler regimens. A research study conducted at the Groningen Research Institute for Asthma and COPD (GRIAC) in the Netherlands concluded that smart inhalers help healthcare professionals by providing guided self-management. It was also stated that several studies have found that electronic inhaler reminders significantly increase medication adherence compared to standard care. This leads to a reduction in hospitalizations, emergency room visits, and associated healthcare costs, benefiting both patients and healthcare systems.
Teva's ProAir Digihaler is the first digital inhaler with built-in sensors, which allows for remote monitoring and adjustment of treatment plans. This became especially important during the COVID-19 pandemic and has since become a standard of care. Smart inhalers empower patients by providing them with tools to manage their condition actively. The ability to track their inhaler usage and symptoms enhances patient engagement in their own care.
Therefore, the increasing demand for smart inhalers has driven innovation in the field, and more companies are investing in research and development, resulting in a wider range of smart inhaler options. However, challenges such as the availability of traditional inhalers as a substitute, stringent regulatory requirements, and compliance standards could impede innovation and rapid expansion of the smart inhalers market. In addition, the potential financial burden on patients, stemming from the higher costs associated with these advanced devices, may pose a restraint, limiting their accessibility, particularly in regions with limited healthcare resources.
Smart Inhalers Market Trends
The Metered Dosage Inhalers (MDI) Segment is Expected to Dominate the Smart Inhalers Market During the Forecast Period
MDIs are inhalation devices that deliver medication in a precisely measured spray or mist. They are propelled by a propellant, and patients should coordinate their breath with inhaler actuation. In most cases, MDIs are preferred due to their familiarity and ease of use. Many pharmaceutical companies and inhaler manufacturers are integrating smart features into MDIs, making them attractive options for patients who are looking to enhance medication adherence and disease management. For instance, AstraZenca's Symbicort Inhaler incorporates inhaler analytics to track usage and provide feedback to patients in order to enhance medication adherence.
Ongoing technological advancements and product approvals for MDI-based smart inhalers are further projected to propel the market. Technological benefits, such as dose counters, inhaler analytics, and integration with mobile apps, contribute to MDIs' dominance in the smart inhalers market. For instance, in February 2022, HeroTracker Sense was designed to improve the lives of patients with chronic respiratory disease caused by COVID-19. It helped track their MDI usage and facilitate improved adherence to their prescribed therapy.
Therefore, the MDI-based smart inhalers segment is expected to dominate over the forecast period due to major factors such as the growing emphasis on patient-centric healthcare, technological advancements enhancing inhaler effectiveness, and rising product approvals involving the integration of digital health solutions into respiratory care. These drivers collectively underscore the promising trajectory of MDIs within the dynamic landscape of smart inhalers.
North America is Expected to Dominate the Smart Inhalers Market
North America is expected to dominate the market owing to factors such as the high prevalence of respiratory diseases, rapid growth in advanced technologies, and robust healthcare infrastructure in the region.
The prevalence of respiratory diseases, such as asthma and COPD, is substantial in North America. As per the Asthma and Allergy Foundation of America, in 2023, more than 27 million people in the United States had asthma, equaling about 1 in 12 people. Moreover, as per the same source, more than 22 million United States adults aged 18 and older had asthma. Furthermore, according to the Centers for Disease Control and Prevention (CDC), cases of asthma in children have declined from 2001 through 2020. However, it was estimated that 50% of children had uncontrolled asthma.
North American companies and research institutions have been at the forefront of developing smart inhaler technology. Key product launches and the high concentration of market players in the United States are some of the factors driving the growth of the smart inhalers market in the country. For example, Propeller Health, a US-based firm, developed a platform with smart inhalers and sensors for asthma and COPD management. The US Food and Drug Administration (FDA) has been actively involved in regulating smart inhaler devices. In 2021, BreatheSuite Inc., a connected respiratory health company in Canada, received the 510(K) clearance from the US FDA for its BreatheSuite metered-dose inhaler (MDI) V1 device.
Therefore, owing to the aforesaid factors, the market studied is anticipated to witness growth in North America.
Smart Inhalers Industry Overview
The smart inhalers market is fragmented due to the presence of several companies operating globally and regionally. The competitive landscape includes an analysis of a few international and local companies that hold market shares and are well known. The key players include Teva Pharmaceutical Industries Ltd, AstraZeneca, GlaxoSmithKline PLC, Novartis AG, ResMed, Adherium, AptarGroup Inc., Findair Sp. z o. o., Boehringer Ingelheim International GmbH, Philip Morris International Inc. (Vectura Group PLC), BreatheSuite Inc., and Cognita Labs.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Prevalence of Respiratory Diseases
- 4.2.2 Technological Advancements Owing to Telehealth and Remote Patient Monitoring
- 4.2.3 Improved Medication Adherence
- 4.3 Market Restraints
- 4.3.1 Availability of Traditional Inhalers as a Substitute
- 4.3.2 High Cost Associated with These Advanced Devices
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Product Type
- 5.1.1 Dry Powder Inhalers (DPI)
- 5.1.2 Metered Dosage Inhalers (MDI)
- 5.2 By Indication
- 5.2.1 Asthma
- 5.2.2 Chronic Obstructive Pulmonary Disorders (COPD)
- 5.2.3 Others
- 5.3 By Distribution Channel
- 5.3.1 Hospital Pharmacies
- 5.3.2 Retail Pharmacies
- 5.3.3 E-commerce
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Teva Pharmaceutical Industries Ltd
- 6.1.2 AstraZeneca
- 6.1.3 GlaxoSmithKline PLC
- 6.1.4 Novartis AG
- 6.1.5 ResMed
- 6.1.6 Adherium
- 6.1.7 AptarGroup Inc.
- 6.1.8 Findair Sp. z o. o.
- 6.1.9 Boehringer Ingelheim International GmbH
- 6.1.10 Philip Morris International Inc. (Vectura Group PLC)
- 6.1.11 BreatheSuite Inc.
- 6.1.12 Cognita Labs
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




