PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1521871

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1521871
America Cancer Immunotherapy - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
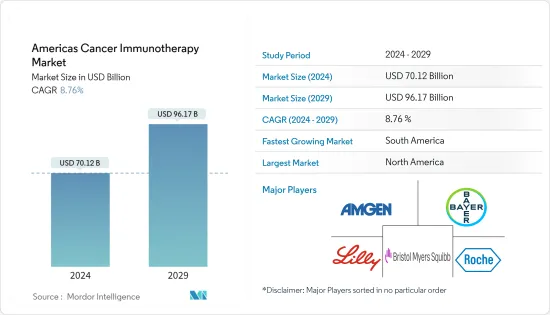
The Americas Cancer Immunotherapy Market size is estimated at USD 70.12 billion in 2024, and is expected to reach USD 96.17 billion by 2029, growing at a CAGR of 8.76% during the forecast period (2024-2029).
The American cancer immunotherapy market is anticipated to witness significant growth opportunities due to the rising burden of cancer, rising R&D activities, and the increasing effectiveness and accuracy of newer therapies. In addition, growing funding activities and several strategic initiatives undertaken by industry participants are projected to accelerate market growth over the study period.
The alarming increase in the burden of cancer across the Americas is expected to bolster the demand for innovative cancer therapies like immunotherapy to treat the condition. For instance, according to the American Cancer Society, the estimated number of new cancer cases ramped up from 1.9 million in 2023 to 2.0 million in 2024. The most common type of cancers diagnosed in 2023 were breast, lung, and hematological malignancies.
Similarly, according to the estimates from the National Cancer Institute of Brazil, around 704 thousand new cancer cases are expected to be diagnosed between 2023 and 2025, except non-melanoma skin cancer. Thus, such an alarming burden of cancer in the region is projected to accelerate the uptake of novel cancer therapies like immunotherapy for the effective treatment of disease.
Thus, the increased burden of cancers has accelerated the adoption of advanced cancer treatment options like immunotherapies. Moreover, several clinical benefits of immunotherapies in cancer and leveraging the role of immunotherapies in cancer management are further expected to accelerate market growth over the study period.
For instance, according to an article published by the International Journal of Molecular Sciences in September 2023, immunotherapy recently emerged as an innovative treatment option for lethal cancers like prostate cancer. It has several clinical benefits, including targeted therapy and improved clinical outcomes. Thus, leveraging the role of immunotherapy in the management of cancers is expected to accelerate market expansion over the study period.
Furthermore, supportive government legislation and growing regulatory approvals are further projected to accelerate industry expansion over the study period. For instance, in March 2024, the United States Food and Drugs Administration (U.S.FDA) approved Bristol Myers Squibb's Opdivo (nivolumab) in combination with gemcitabine and cisplatin for the first-line treatment of metastatic urothelial carcinoma in adults. This approval marked one of the key immunotherapy-chemotherapy combinations approved for patients with metastatic urothelial carcinoma.
Similarly, in July 2022, the US FDA accepted Genentech's Biologics License Application (BLA) and granted Priority Review for mosunetuzumab, a T-cell-engaging bispecific antibody. The immunotherapy drug is a first-in-class CD20xCD3 T-cell-engaging bispecific antibody intended for the management of relapsed or refractory (R/R) follicular lymphoma (FL) in adult patients. Thus, such growing regulatory approvals are projected to foster market growth over the study period.
Moreover, in March 2024, Caring Cross and Fundacao Oswaldo Cruz collaborated to develop domestic production of stem cell gene therapies and CAR-T cell therapies for various types of cancers, including leukemia, and lymphoma.
America Cancer Immunotherapy Market Trends
Breast Cancer Segment is Expected to Exhibit a Significant Market Growth Over the Forecast Period
The segment includes cancer immunotherapies for treating breast cancer, which is one of the most common cancers among women and most likely to affect women over the age of 50.
The segment is anticipated to grow during the forecast period owing to the high burden of breast cancer and increasing demand for cancer immunotherapies. For instance, according to the American Cancer Society, the burden of breast cancer increased significantly in the United States, and the total number of cases ramped from 300,590 in 2023 to 313,510 in 2024.
Similarly, the higher burden of breast cancer in South American countries is further expected to accelerate segment expansion over the study period. For instance, according to 2023 updated data from the Global Cancer Observatory, around 21,631 new breast cancer cases were diagnosed in Argentina in the year 2022, which is expected to increase over coming years, and the burden of breast cancer was much higher than other cancer types in Argentina. Thus, the fostering demand for breast cancer is expected to fuel segment growth over the study period.
The segment growth is expected to be driven by increased efforts from public and private organizations to foster awareness levels about breast cancer and its management. For instance, in December 2023, Breast Cancer Canada launched a portal, Progress CONNECT, to promote awareness about breast cancer along with recent advances in breast cancer treatment.
In addition, in June 2022, Daiichi Sankyo Brazil undertook a free breast cancer screening for women in Brazil's Amazon region. This initiative further helped to strengthen awareness levels of breast cancer among women. Thus, such initiatives are expected to foster segment growth over the study period.
Moreover, the recent developments by the market players are expected to boost the availability of more breast cancer immunotherapy, which is projected to facilitate segment uptake over the study period. For instance, in April 2024, the United States Food and Drug Administration (U.S.FDA) approved AstraZeneca and Daiichi Sankyo's Enhertu (trastuzumab deruxtecan) for the treatment of metastatic HER2-positive breast cancer in adults.
Similarly, in August 2022, Memorial Sloan Kettering Cancer Center received approval from the United States Food and Drug Administration for its first targeted therapy, Trastuzumab Deruxtecan (T-DXd). The cancer immunotherapy is intended for the management of HER2-low breast cancer patients.
Therefore, the above-mentioned factors, such as the alarming rise in breast cancer, increasing awareness for breast cancer, and several strategic initiatives undertaken by market players, are anticipated to drive the growth of the studied segment.
North America is Expected to Witness Significant Growth Over the Forecast Period
North America is expected to witness significant growth over the forecast period owing to the advanced medical infrastructure, the high burden of cancer, and the strategic initiatives the market players took.
The increase in cancer cases across the region is expected to elevate the demand for cancer immunotherapy, propelling market growth. For instance, according to the data published by the American Cancer Society in 2024, the country reported around 62,770 cases of leukemia in 2024 as compared to 59,610 in 2023. Thus, the alarming increase in the burden of cancer in the region is projected to facilitate market expansion over the study period.
North America is a region with a robust healthcare infrastructure and a supportive research environment. In recent years, the research activities for novel cancer treatment development have increased, and this is projected to accelerate market growth over the study period. For instance, in March 2024, Purdue University, United States, developed advanced nanoparticles to enhance the systemic delivery of cancer immunotherapy.
In addition, in May 2024, the scientists of Washington University School of Medicine received a grant of USD 5 million from the Leukemia & Lymphoma Society (LLS) to develop novel immunotherapies for various blood cancers. Such research activities are expected to accelerate regional market growth over the study period.
Moreover, the recent developments by the market players in the country are expected to boost the market growth in the region. For instance, in March 2023, Hoffmann-La Roche Limited (Roche Canada) received authorization from Health Canada for its cancer therapy, COLUMVI (glofitamab for injection), intended for the management of relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Thus, such developments undertaken by industry participants are expected to foster market growth over the study period.
Thus, the above-mentioned factors, such as the increased burden of cancer, growing funding/grants, and several strategic initiatives undertaken by industry participants, are projected to accelerate market growth in the region over the study period.
America Cancer Immunotherapy Industry Overview
America's cancer immunotherapy market is fragmented in nature due to the presence of several market players operating regionally. Companies are undertaking several strategic initiatives to strengthen their business avenues, such as investing in research and product development. Some of the leading players operating in the market include Amgen Inc., Bayer AG, Bristol-Myers Squib Eli Lilly and Company, and F. Hoffman La Roche Ltd.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising R&D Activities and Increasing Effectivity and Accuracy Of Newer Therapies
- 4.2.2 High Burden of Cancer
- 4.3 Market Restraints
- 4.3.1 Side Effects Associated With the Immunotherapy
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market size by Value in USD)
- 5.1 By Therapy Type
- 5.1.1 Monoclonal Antibodies
- 5.1.2 Cancer Vaccines
- 5.1.3 Immunomodulators
- 5.1.4 Immune Check Point Inhibitors
- 5.1.5 Other Therapy Types
- 5.2 By Application
- 5.2.1 Prostate Cancer
- 5.2.2 Breast Cancer
- 5.2.3 Skin Cancer
- 5.2.4 Lung Cancer
- 5.2.5 Other Applications
- 5.3 By End Users
- 5.3.1 Hospitals and Clinics
- 5.3.2 Cancer Research Centers
- 5.3.3 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 South America
- 5.4.2.1 Brazil
- 5.4.2.2 Argentina
- 5.4.2.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Amgen Inc.
- 6.1.2 Astellas Pharma Inc.
- 6.1.3 AstraZeneca
- 6.1.4 Bayer AG
- 6.1.5 Bristol-Myers Squibb Company
- 6.1.6 Eli Lilly and Company
- 6.1.7 F. Hoffman La Roche Ltd
- 6.1.8 Merck and Co. Inc.
- 6.1.9 Novartis AG
- 6.1.10 Y-mAbs Therapeutics, Inc
- 6.1.11 bluebird bio, Inc.
- 6.1.12 Pfizer Inc.
- 6.1.13 Gilead Sciences
- 6.1.14 GSK plc
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




