PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851271

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851271
Medical Devices Packaging - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The medical device packaging market size is USD 42.41 billion in 2025 and is forecast to reach USD 56.83 billion by 2030, advancing at a 6.03% CAGR.
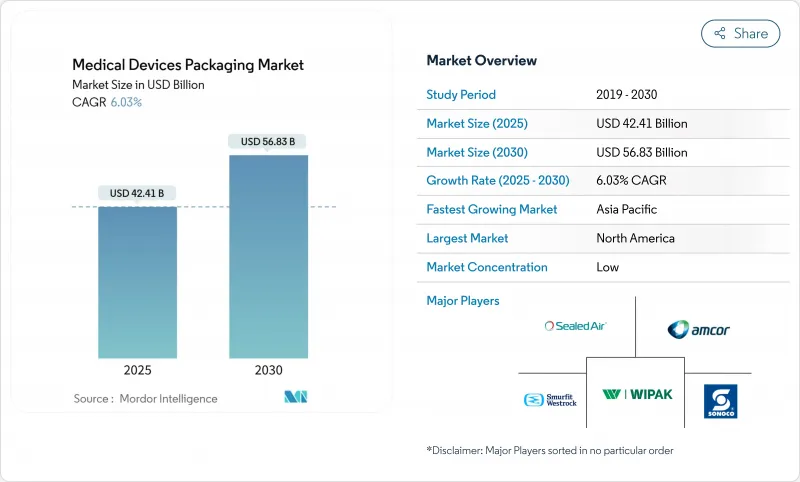
This momentum is underpinned by tighter sterility standards, rapid adoption of smart-label technologies, and a steady pipeline of minimally invasive and wearable devices that demand high-performance barrier formats. Material innovation remains a core growth lever because polymers such as cyclic olefin copolymers and liquid-crystal polymers withstand high-temperature sterilization while enabling RFID integration. Contract sterilization networks continue to expand, which raises demand for standardized primary packs that perform consistently across multiple facilities. At the same time, raw-material cost volatility and limited ethylene oxide capacity are forcing converters to redesign packs for alternative sterilization methods, creating both cost pressures and innovation windows. Geographically, North America preserves market leadership yet Asia-Pacific delivers the fastest incremental revenue, thanks to hospital build-outs in China and India and policy-backed local manufacturing.
Global Medical Devices Packaging Market Trends and Insights
Rising Demand for Extended-Shelf-Life Formats
Healthcare providers want devices that remain usable for five to seven years because pandemic preparedness and rural outreach stretch replenishment cycles. Barrier films incorporating ethylene vinyl alcohol and metallized polyester now deliver that longevity, and DuPont's 2025 Costa Rica expansion-adding 16,000 ft2 solely for sterile Tyvek packs-underscores the global push for superior barriers. Accelerated-aging and real-time stability protocols are becoming routine, shifting material choice toward premium polymers despite higher purchase prices.
Growth in Minimally-Invasive and Wearable Devices
New laparoscopic tools and connected wearables feature complex geometries and sensitive electronics that require gentle yet sterile containment. Thermoformed trays with custom cavities dominate for small surgical sets, whereas flexible pouches embedded with temperature-sensitive inks suit smart patches destined for home use. Packaging scientists are also mitigating adhesive migration to ensure skin safety for wearable sensors, a challenge cited by device integrators interviewed across APAC clinics.
Regulatory Compliance Cost Burden
Validation outlays have risen 25-30% under the EU MDR because each pack configuration must pass biocompatibility, accelerated-aging, and distribution-simulation batteries. Smaller converters are consolidating platforms to curb test repetition, yet this can compromise application fit. The complexity fuels a niche consulting segment that guides dossier compilation at premium fees.
Other drivers and restraints analyzed in the detailed report include:
- Stricter Global Sterility Regulations
- Integration of RFID/UDI Smart-Label Traceability
- Volatile Medical-Grade Polymer Prices
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Plastics retained 55.23% of the medical device packaging market in 2024, and the segment leads growth with an 8.22% CAGR through 2030. Advanced polymers such as cyclic olefin copolymers enable steam and plasma sterilization without warping, whereas liquid-crystal polymers support smart circuitry embedment. As a result, the medical device packaging market records steady migration away from legacy glass and metal for primary packs. Paperboard continues in secondary roles where cost efficiency trumps barrier performance. Bio-based plastics, though under 5% of volume, post double-digit expansion riding on hospital sustainability scorecards and Asia-Pacific's emergence as a biopolymer hub.
Rising demand for multi-layer films that withstand gamma and e-beam sterilization fuels capital investment in co-extrusion lines across the United States and Malaysia. Polymer suppliers leverage vertical integration to guarantee medical-grade resin purity, positioning themselves as assured partners for validated barrier systems. These dynamics are expected to uphold plastics' commanding share of the medical device packaging market through the forecast horizon.
Pouches and bags delivered 36.32% of 2024 revenues owing to their versatility across single-use disposables and electronic catheters. However, kit complexity in orthopedic and cardiovascular interventions lifts demand for rigid boxes and cartons, causing this format to pace at a 9.32% CAGR. Consequently, multilayer cartons lined with Tyvek are gaining share where outer rigidity and inner sterility must coexist. Trays remain indispensable for delicate scopes, while thermoformed blisters secure small items such as diagnostic strips.
Third-party sterilizers increasingly request flat pouches equipped with optimized peelability that speeds operating-room presentation. In parallel, carton suppliers integrate transparent apertures so clinicians can verify instrument sets without breaking seals. These usability tweaks reinforce product-type diversification within the medical device packaging market.
The Medical Device Packaging Market Report is Segmented by Material (Plastics, Paper and Paperboard, and More), Product Type (Pouches and Bags, and More), Application (Sterile Packaging, Non-Sterile Packaging, Active/Smart Packaging), End User (Hospitals and Clinics, Diagnostic and Imaging Centers, and More ), Packaging Level (Primary, Secondary, Tertiary), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led with 35.43% share in 2024, propelled by the United States' robust device innovation base and FDA's clear validation pathways. Canadian hospital-modernization programs boost demand for cost-effective packs, while Mexico's maquiladora clusters integrate cross-border packaging lines that meet both FDA and COFEPRIS standards. Material circularity targets drive trial runs of recyclable high-density polyethylene film across several US hospital systems, initiatives supported by Amcor's expanded closed-loop projects.
Asia-Pacific advances at a 10.83% CAGR, anchored by China, Japan, and India. China's forecast USD 210 billion device market prompts label-localization investments and UDI-compliant printing, drawing converters into provincial hubs such as Suzhou. Japan's ageing demographic multiplies the need for home-use kits preserved in long-life pouches, while India's Make-in-India incentives attract joint ventures that erect extrusion and die-cutting capacity near Ahmedabad.
Europe remains mature yet innovation-driven. The EU MDR compels multi-language labeling and proof of recyclability, and Germany's orthopedic cluster partners with packaging labs to prototype mono-material sterility systems. Post-Brexit divergence obliges dual certification for exporters targeting both EU and UK markets. The Middle East and Africa record steady gains as Gulf states commission new hospitals, whereas South America's growth stems from Brazil's domestic pacemaker lines that now source Tyvek trays from local converters.
- Amcor plc
- DuPont de Nemours Inc.
- Smurfit WestRock
- Mitsubishi Chemical Group
- Sonoco Products Company
- Technipaq Inc.
- SteriPack Group
- Riverside Medical Packaging
- Wipak Group
- 3M Company
- Sealed Air Corporation
- Constantia Flexibles
- Klockner Pentaplast Group
- Placon Corporation
- West Pharmaceutical Services
- Nelipak Healthcare Packaging
- Oliver Healthcare Packaging
- Tekni-Plex
- Multivac Group
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising demand for extended-shelf-life formats
- 4.2.2 Growth in minimally-invasive and wearable devices
- 4.2.3 Stricter global sterility regulations (ISO 11607, EU MDR, FDA)
- 4.2.4 Integration of RFID/UDI smart-label traceability
- 4.2.5 Carbon-footprint disclosure pushing mono-materials
- 4.2.6 Expansion of outsourced contract sterilization networks
- 4.3 Market Restraints
- 4.3.1 Regulatory compliance cost burden
- 4.3.2 Volatile medical-grade polymer prices
- 4.3.3 Scarcity of bio-based and PCR resins
- 4.3.4 Cold-chain e-commerce seal-failure recalls
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Sustainability Trends and LCA Impact
- 4.8 Porter's Five Forces Analysis
- 4.8.1 Bargaining Power of Suppliers
- 4.8.2 Bargaining Power of Buyers
- 4.8.3 Threat of New Entrants
- 4.8.4 Threat of Substitutes
- 4.8.5 Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Material
- 5.1.1 Plastics
- 5.1.2 Paper and Paperboard
- 5.1.3 Metals and Foils
- 5.1.4 Glass
- 5.1.5 Bio-based Plastics
- 5.2 By Product Type
- 5.2.1 Pouches and Bags
- 5.2.2 Trays
- 5.2.3 Boxes and Cartons
- 5.2.4 Blister Packs
- 5.2.5 Other Product Types
- 5.3 By Application
- 5.3.1 Sterile Packaging
- 5.3.2 Non-sterile Packaging
- 5.3.3 Active / Smart Packaging
- 5.4 By End User
- 5.4.1 Hospitals and Clinics
- 5.4.2 Diagnostic and Imaging Centers
- 5.4.3 Home Healthcare
- 5.4.4 Contract Manufacturing and Sterilization Orgs
- 5.5 By Packaging Level
- 5.5.1 Primary
- 5.5.2 Secondary
- 5.5.3 Tertiary
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Russia
- 5.6.2.7 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 India
- 5.6.3.3 Japan
- 5.6.3.4 South Korea
- 5.6.3.5 Australia and New Zealand
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 Middle East
- 5.6.4.1.1 United Arab Emirates
- 5.6.4.1.2 Saudi Arabia
- 5.6.4.1.3 Turkey
- 5.6.4.1.4 Rest of Middle East
- 5.6.4.2 Africa
- 5.6.4.2.1 South Africa
- 5.6.4.2.2 Nigeria
- 5.6.4.2.3 Egypt
- 5.6.4.2.4 Rest of Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share, Products and Services, Recent Developments)
- 6.4.1 Amcor plc
- 6.4.2 DuPont de Nemours Inc.
- 6.4.3 Smurfit WestRock
- 6.4.4 Mitsubishi Chemical Group
- 6.4.5 Sonoco Products Company
- 6.4.6 Technipaq Inc.
- 6.4.7 SteriPack Group
- 6.4.8 Riverside Medical Packaging
- 6.4.9 Wipak Group
- 6.4.10 3M Company
- 6.4.11 Sealed Air Corporation
- 6.4.12 Constantia Flexibles
- 6.4.13 Klockner Pentaplast Group
- 6.4.14 Placon Corporation
- 6.4.15 West Pharmaceutical Services
- 6.4.16 Nelipak Healthcare Packaging
- 6.4.17 Oliver Healthcare Packaging
- 6.4.18 Tekni-Plex
- 6.4.19 Multivac Group
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-need Assessment




