PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1907351

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1907351
Intraocular Lens - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2026 - 2031)
The intraocular lens market size in 2026 is estimated at USD 7.34 billion, growing from 2025 value of USD 6.89 billion with 2031 projections showing USD 10.09 billion, growing at 6.57% CAGR over 2026-2031.
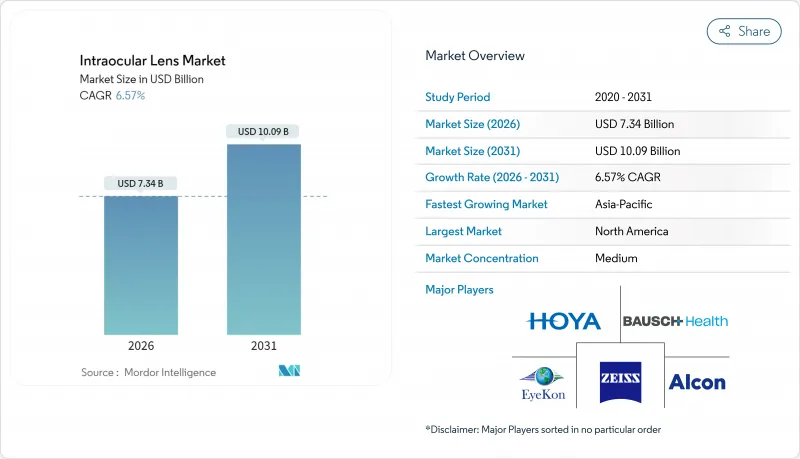
Premium lens innovations, the growing pool of older adults, and a shift toward outpatient surgical models anchor this expansion. An enlarging 65+ demographic brings a steady cataract case flow, while presbyopia-correcting and light-adjustable designs let surgeons match rising lifestyle expectations for spectacle independence. Silicone and next-generation hydrophobic acrylic materials reduce inflammatory events, encouraging surgeons to discuss premium upgrades more confidently. Asia-Pacific's medical-tourism corridors widen access to advanced lenses, and office-based suites improve provider economics, creating new procedure capacity. Competitive intensity stays high because every product cycle that lifts visual quality or lowers dysphotopsia quickly resets surgeon preferences.
Global Intraocular Lens Market Trends and Insights
Aging-linked rise in global cataract procedures
Cataract incidence parallels population longevity. Individuals over 80 have a 70% likelihood of developing lenticular opacity, and many expect high-quality vision for continued digital engagement and later-life employment. Ambulatory surgery centers already log cataract as their largest case type, representing 19% of ASC volume in 2024. Capacity pressure in Asia-Pacific magnifies because demographic aging outpaces clinic build-out, so providers increasingly adopt high-throughput models that pair phaco units with preloaded lens systems.
Surge in adoption of premium / presbyopia-correcting IOLs
Premium penetration rose from 15.5% in 2019 to 18.5% in 2021 despite reimbursement gaps. Light-adjustable optics let surgeons refine power post-op, shrinking the risk of residual refractive error. Alcon holds more than 60% of this segment on the strength of PanOptix and Vivity families. Enhanced monofocal designs such as Tecnis Eyhance extend depth without the photic issues of diffractive rings, broadening eligibility for patients wary of halo or glare.
High out-of-pocket cost and uneven reimbursement for premium IOLs
Patients often pay USD 1,500-3,000 per eye because CMS lists no New Technology IOL codes, creating a two-tier access model. The financial load includes diagnostic aberrometry and follow-up adjustments, deterring price-sensitive candidates. International travel can lower the bill, yet quality assurance varies across facilities.
Other drivers and restraints analyzed in the detailed report include:
- Rapid product cycles: light-adjustable and AI-designed lenses
- Growth of refractive lens exchange in the 40-60 age cohort
- Post-operative dysphotopsia concerns limiting surgeon uptake
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Monofocal lenses retained volume leadership with 62.68% intraocular lens market share in 2025. Premium categories, spanning trifocal, toric, EDOF, and accommodating designs, post a 7.16% CAGR that surpasses baseline cataract growth. Demand stems from patients who prioritize uncorrected near vision and from surgeons promoting refractive outcomes as part of cataract management. Multifocal options like PanOptix yield high spectacle independence and fewer halos than early bifocal models. Toric monofocals correct up to 4 D of corneal cylinder and have become routine in eyes with >=1 D astigmatism. EDOF optics such as Tecnis Symfony trade some near acuity for reduced photic side effects, fitting patients skeptical about diffractive rings. Accommodating prototypes including Juvene target >=3.5 D amplitude, aiming to replicate physiologic focus change, a milestone market observers expect to unlock accelerated premium conversion.
Surgical centers bundle presbyopia-correcting lenses with femtosecond assisted capsulotomy to enhance centration, while topographers refine pre-op planning for toric axis alignment. Clinicians report that post-refractive-surgery patients often prefer premium solutions because light-adjustable technology can fine-tune residual error. The premium tier extends revenue per procedure, helping clinics offset reimbursement headwinds and encouraging investment in advanced diagnostics.
Hydrophobic acrylic continues to underpin almost half of the intraocular lens market size thanks to foldability, capsular biocompatibility, and glistening-resistant formulations. Surface-engineered variants like Clareon boost water content to aid clarity yet maintain low calcification risk. Silicone's 7.05% CAGR signals a renaissance; higher purity grades minimize inflammatory cell adhesion, making these lenses attractive in uveitic eyes. Newer silicone optics incorporate UV-blocking chromophores and can accept femtosecond power refinement post-implant. Hydrophilic acrylic now represents 28.90% of units, rehabilitated by cross-linked polymers and anti-calc coatings that preserve clarity in diabetic vitreous environments. PMMA use declines except in trauma cases that benefit from rigid stability.
Material research focuses on reducing posterior capsule opacification through edge-design micro-texturing and exploring bioresorbable haptics that vanish after capsular fibrosis secures the optic. Suppliers stress dual-sourcing of raw monomers because pandemic disruptions revealed dependency risks in hydrophobic acrylic chains.
The Intraocular Lens Market is Segmented by Product Type (Monofocal IOL, Premium IOL [Multifocal, Toric, and More], and More), Material (Hydrophobic Acrylic and More), End User (Hospitals, Ambulatory Surgery Centers, and More), Application (Cataract, Presbyopia, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led the intraocular lens market in 2025 with 41.76% revenue because Medicare covers baseline cataract surgery and patients can self-fund upgrades. Premium penetration tops 21.80% in the United States, and ophthalmology practices deploy heavy advertising to attract RLE candidates. The intraocular lens market size for the region is projected to surpass USD 4.18 billion by 2031 at 5.55% CAGR, supported by rapid adoption of office-based surgical suites and adjustable-lens platforms.
Asia-Pacific records the fastest 7.22% CAGR thanks to demographic aging, expanding middle-class spending power, and thriving medical-tourism clusters. Thailand and Singapore package premium IOL surgery with three-day recovery stays, drawing inbound volumes that lift average selling prices. China continues to scale cataract capacity, yet premium uptake remains below 9.75%, signaling sizable headroom for growth once income and reimbursement levels rise. India's high-volume hubs replicate the Aravind model that combines efficiency with modular pricing, bringing premium adoption into reach for urban consumers.
Europe features mature reimbursement but strong sustainability norms. Regulators encourage reduced-plastic delivery systems, prompting lens makers to trial bio-derived cartridge polymers. Germany and Spain report premium penetration near 19.70%, while the United Kingdom remains conservative amid National Health Service budget constraints. CE-marked launches such as Clareon Vivity in 2025 widen presbyopia-correction choice for surgeons.
The Middle East and Africa expand from a lower base as public-private partnerships build specialty eye hospitals in Gulf states and North Africa. Wealthy patients often fly to Europe or Asia for premium surgery, but new centers in Dubai and Riyadh aim to reverse outbound flow.
South America benefits from price-arbitrage seekers from North America; Brazil's private insurers now reimburse certain EDOF lenses, lifting regional demand.
- Alcon
- Johnson & Johnson
- Bausch + Lomb Corp.
- Carl Zeiss
- Hoya Corp.
- Staar Surgical Co.
- Rayner Group
- HumanOptics Holding AG
- Lenstec
- PhysIOL (BVI)
- Ophtec BV
- SAV-IOL SA
- Aurolab
- Medicontur Medical Engineering
- Santen Pharmaceutical Co.
- Biotech Healthcare
- EyeKon Medical
- Rodenstock Group
- Visioncare Ophthalmic Technologies
- Hanita Lenses
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Aging-Linked Rise in Global Cataract Procedures
- 4.2.2 Surge In Adoption of Premium / Presbyopia-Correcting IOLs
- 4.2.3 Rapid Product Cycles: Light-Adjustable & AI-Designed Lenses
- 4.2.4 Growth Of Refractive-Lens-Exchange (RLE) In the 40-60 Age Cohort
- 4.2.5 Medical-Tourism Hubs Lowering Procedure Costs
- 4.2.6 Pre-Loaded, Single-Use IOL Systems Easing OR Bottlenecks
- 4.3 Market Restraints
- 4.3.1 High Out-of-Pocket Cost & Patchy Reimbursement for Premium IOLs
- 4.3.2 Post-Operative Dysphotopsia Concerns Limiting Surgeon Uptake
- 4.3.3 Supply-Chain Dependence on Specialty Hydrophobic Acrylics
- 4.3.4 Sustainability Pressures on Single-Use Plastics in Lens Delivery
- 4.4 Regulatory Landscape
- 4.5 Technological Outlook
- 4.6 Porters Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers/Consumers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product Type
- 5.1.1 Monofocal IOL
- 5.1.2 Premium IOL
- 5.1.2.1 Multifocal
- 5.1.2.2 Toric
- 5.1.2.3 Accommodating
- 5.1.3 Phakic Intraocular Lens (PIOL)
- 5.1.4 Others
- 5.2 By Material
- 5.2.1 Hydrophobic Acrylic
- 5.2.2 Hydrophilic Acrylic
- 5.2.3 Silicone
- 5.2.4 Polymethyl-methacrylate (PMMA)
- 5.2.5 Others
- 5.3 By End User
- 5.3.1 Hospitals
- 5.3.2 Ambulatory Surgery Centers
- 5.3.3 Ophthalmology Clinics
- 5.3.4 Others
- 5.4 By Application
- 5.4.1 Cataract
- 5.4.2 Presbyopia
- 5.4.3 Corneal Disorders
- 5.4.4 Others
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Alcon Inc.
- 6.3.2 Johnson & Johnson Vision
- 6.3.3 Bausch + Lomb Corp.
- 6.3.4 Carl Zeiss Meditec AG
- 6.3.5 Hoya Corp.
- 6.3.6 Staar Surgical Co.
- 6.3.7 Rayner Group
- 6.3.8 HumanOptics Holding AG
- 6.3.9 Lenstec Inc.
- 6.3.10 PhysIOL (BVI)
- 6.3.11 Ophtec BV
- 6.3.12 SAV-IOL SA
- 6.3.13 Aurolab
- 6.3.14 Medicontur Medical Engineering
- 6.3.15 Santen Pharmaceutical Co.
- 6.3.16 Biotech Healthcare
- 6.3.17 Eyekon Medical
- 6.3.18 Rodenstock Group
- 6.3.19 Visioncare Ophthalmic Technologies
- 6.3.20 Hanita Lenses
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




