PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1258776

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1258776
Next-Generation Immunology Drugs Market - Growth, Trends, and Forecasts (2023 - 2028)
The Next-Generation Immunology Drugs Market is expected to register a CAGR of 6.1% over the forecast period.
The COVID-19 pandemic had a significant impact on the market studied. During the pandemic, immunology research was increased to understand the effects of COVID-19 on immunity and cope with the severe effects of the same. For instance, the University of Oxford Immunology Network conducted research on COVID-19 in early 2021 that was focused on humoral immunity, deep phenotyping, the inflammatory response, and cell immunity. Additionally, research in the area of immunology drugs for COVID-19 increased during the pandemic. For instance, JAK and BTK inhibitors were immunomodulatory drugs that were evaluated to treat COVID-19. GM-CSF Inhibitors. The rising research on immunology drugs to combat the COVID-19 disease fueled the market's growth. However, currently, the market has reached its pre-pandemic nature and is expected to witness significant growth over the forecast period.
Some of the key factors propelling market growth include the rising burden of chronic diseases, increasing research in the area of next-generation drugs, and an increased focus on targeted therapies for certain diseases.
The market for next-generation immunology drugs is expected to witness a healthy growth due to the rising prevalence of chronic diseases like asthma, allergic conditions, cancer, and multiple sclerosis. For instance, a January 2023 report from the American Cancer Society indicated that by the year 2040, there will be more than 16.3 million cancer-related deaths and over 27.5 million new cases worldwide due to population growth and aging. The burden will probably increase in the future because risk factors including smoking, eating poorly, and not exercising are becoming more prevalent in economically developing countries. With growing chronic diseases, pharmaceutical companies are focusing more on R&D and developing novel drugs, which is contributing to market growth.
Furthermore, the key activities undertaken by the various companies are supporting market growth. For instance, in November 2022, Human Immunology Biosciences announced that it had raised USD 120 million to develop targeted treatments for autoimmune and allergic diseases. The company developed two drug candidates, called felzartamab and HIB210, that were licensed from the German biotechnology company MorphoSys. Both are in clinical trials, with the former in Phase 2 testing for two rare kidney diseases and the latter in Phase 1. Hence, owing to the increasing focus of companies on developing such novel drugs, it is believed that the market studied is likely to witness strong growth. However, the high cost of next-generation drugs and stringent regulatory policies are some of the factors that are expected to restrain market growth.
Next-Generation Immunology Drugs Market Trends
Cancer is Expected to Witness Significant Growth Over the Forecast Period
The increasing burden of cancer across the globe coupled with the growing research on novel therapies for cancer treatment are expected to fuel the segment's growth. Immunotherapies come in a variety of forms and are used to treat cancer. Immune checkpoint inhibitors, T-cell cell therapy, cancer vaccines, and immune system modulators are a few examples of immunotherapies. Several cancer types and their later stages are treated with these medicines. Rituxan, Yervoy, Adcetris, and Zevalin are a few of the widely used immunotherapy medications.
New drug launches are accelerating segment growth. For instance, in December 2022, the US FDA granted approval to adagrasib (Krazati, Mirati Therapeutics, Inc.), a RAS GTPase family inhibitor, for adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC). Additionally, in April 2022, the US FDA approved a combination of two immunotherapy drugs for the treatment of some people with advanced melanoma. The combination consists of relatlimab and nivolumab (Opdivo) and will be marketed under the name Opdualag. With the launch of such immunology drugs for cancer treatment, the segment is believed to witness significant growth over the forecast period.
Furthermore, one of the major concerns is the rising global cancer burden. Key countries like the United States, Canada, the United Kingdom, and India are experiencing the burden of the disease. For instance, as per the data from the Canadian Cancer Statistics report published in February 2022, it was found that there were 233,900 people expected to be diagnosed with cancer in the year 2022. The report further stated that breast, prostate, and colorectal cancers are the most prevalent cancers. Combined, they account for almost half of all prevalent cases. With the high number of cancer cases in countries like Canada, there is an increasing amount of research for the development of new drugs, which is boosting the segment's growth.
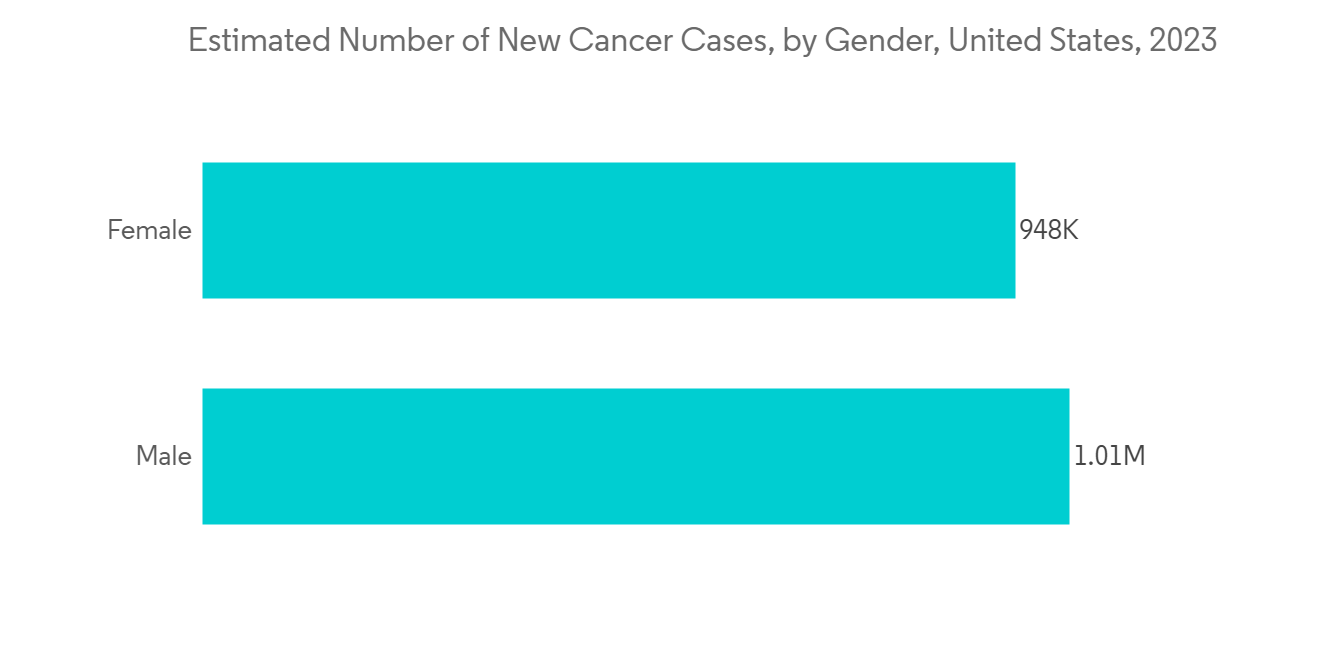
North America to Hold the Largest Market Share Over the Forecast Period
North America is one of the crucial markets for next-generation immunology drugs. In this region, the United States is expected to hold a major share due to its highly developed pharmaceutical R&D sector and the presence of key companies operating in the market studied.
The increasing number of cancer cases is creating tremendous opportunities for market players. The major market players are focusing on R&D activities to launch new and reliable treatments in the market. For instance, in March 2021, the United States Food and Drug Administration approved Bristol-Myers Squibb and Bluebird Bio's Abecma (idecabtagene vicleucel). Abecma is a genetically engineered autologous T cell immunotherapy directed to target the B-cell maturation antigen (BCMA) to treat adult patients with relapsed or refractory multiple myeloma. The rising product launches by the players in the United States lead to further adoption and are thereby expected to propel the market growth in this region.
Additionally, new product launches in the area of next-generation drugs are propelling market growth. For instance, in May 2022, Novartis announced the US FDA's approval of Kymriah (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy. Additionally, in April 2022, Kite Pharma Inc. received product approval from the US FDA for its axicabtagene ciloleucel (Yescarta) for adult patients with large B-cell lymphoma (LBCL). As a result of the numerous new product launches, the United States is expected to experience significant growth during the forecast period.
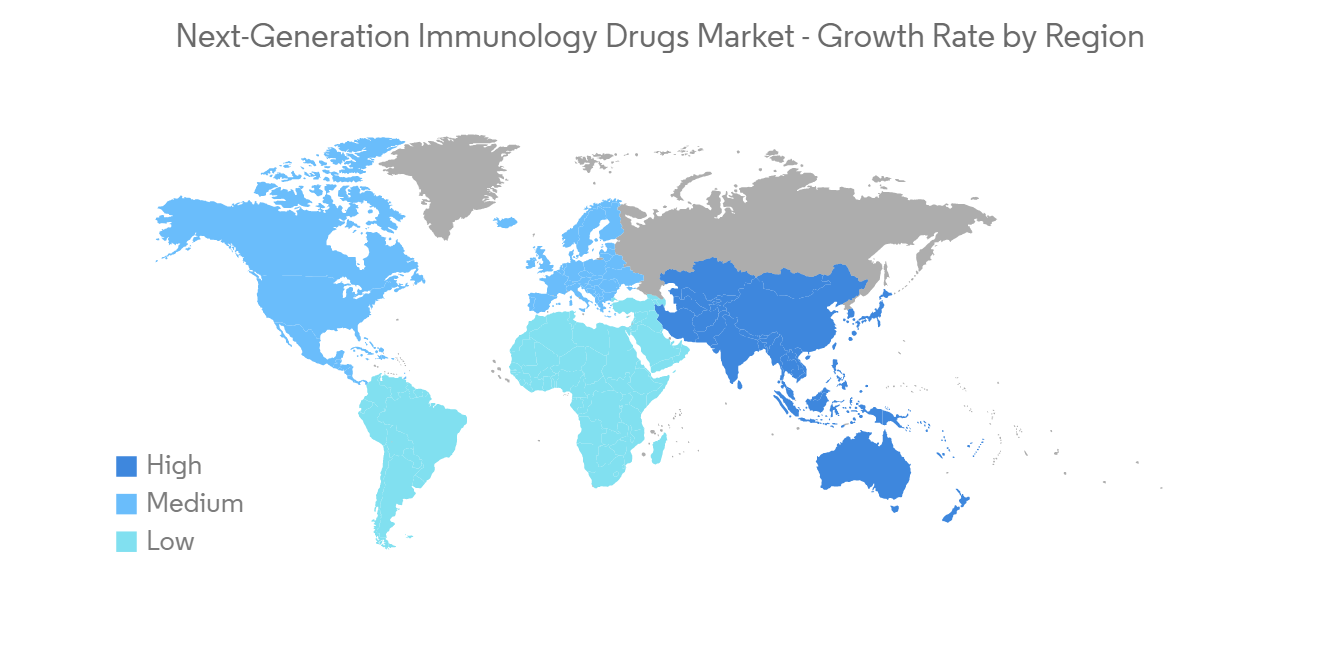
Next-Generation Immunology Drugs Industry Overview
The next-generation immunology drugs market is moderately competitive and consists of several major players. In terms of market share, few of the major players currently dominate the market. The market players are focusing on new product launches, R&D, and expansions to gain a competitive advantage. Some of the market's major players include AbbVie Inc., Amgen Inc., AstraZeneca, GlaxoSmithKline PLC, Novartis AG, and F. Hoffmann-La Roche AG.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Burden of Chronic Diseases
- 4.2.2 Increasing Research in the Area of Next Generation Drugs
- 4.2.3 Increasing Focus on Targeted Therapies
- 4.3 Market Restraints
- 4.3.1 High Cost of Next Generation Drugs
- 4.3.2 Stringent Regulatory Policies
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Drug Class
- 5.1.1 Small Molecules
- 5.1.2 Antibodies
- 5.1.3 Cell Based Therapies
- 5.1.4 Other Drug Classes
- 5.2 By Therapeutic Area
- 5.2.1 Cancer
- 5.2.2 Autoimmune Diseases
- 5.2.3 Infectious Diseases
- 5.2.4 Inflammatory Diseases
- 5.2.5 Other Therapeutic Areas
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Pfizer Inc
- 6.1.2 Abbvie Inc.
- 6.1.3 Johnson and Johnson
- 6.1.4 F. Hoffmann-La Roche Ltd.
- 6.1.5 Amgen Inc.
- 6.1.6 Novartis AG
- 6.1.7 Astellas Pharma Inc.
- 6.1.8 UCB SA
- 6.1.9 Bristol-Myers Squibb Company
- 6.1.10 Merck & Co., Inc.
- 6.1.11 Eli Lilly and Company
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




