PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1699415

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1699415
Fabry Disease Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034
The Global Fabry Disease Treatment Market was valued at USD 2.3 billion in 2024 and is projected to grow at a CAGR of 8.2% during 2025-2034. The increasing demand for highly effective, targeted, and specific treatments for Fabry disease is driving market expansion worldwide. The condition, a rare genetic disorder caused by mutations in the GLA gene, leads to the buildup of harmful fatty substances in various organs, resulting in severe complications affecting the kidneys, heart, and nervous system. As awareness of Fabry disease continues to grow, healthcare providers and pharmaceutical companies are focusing on developing innovative therapies that address both the symptoms and underlying causes. With ongoing research and clinical advancements, new treatment options are emerging, giving patients improved outcomes and an enhanced quality of life. Increased funding from both public and private sectors, along with collaborations among biopharmaceutical companies, is expected to accelerate innovation in this market. Regulatory support, along with fast-track designations for novel therapies, is further expediting drug approvals, creating lucrative opportunities for industry participants. The competitive landscape is evolving, with companies investing heavily in research and development to bring more effective and accessible treatments to market.
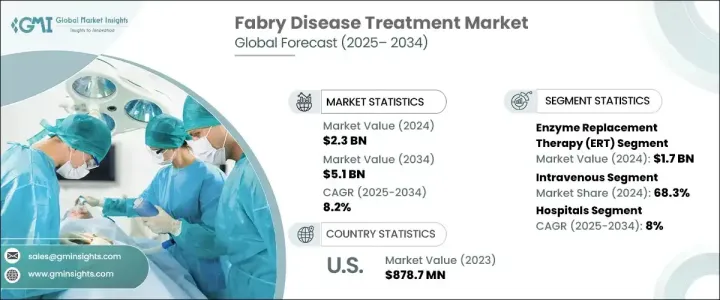
The market is categorized into several treatment options, with enzyme replacement therapy (ERT) leading the way. ERT was valued at USD 1.7 billion in 2024 and continues to dominate due to its established role as the standard treatment for Fabry disease. This therapy works by replacing the deficient enzyme alpha-galactosidase A, an essential component in managing the disease and preventing progressive organ damage. The rising prevalence of Fabry disease has directly increased the demand for ERT, with more patients gaining access to treatment due to improved diagnostic methods. Next-generation sequencing and enzyme assays have revolutionized Fabry disease diagnosis, enabling earlier and more accurate detection, which is further fueling the adoption of ERT. Ongoing research into next-generation ERT formulations with extended half-lives and improved efficacy is expected to sustain growth in this segment.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.3 Billion |
| Forecast Value | $5.1 Billion |
| CAGR | 8.2% |
The market also distinguishes treatments based on their route of administration, including intravenous and oral options. Intravenous treatments accounted for a 68.3% share in 2024 and are expected to continue growing at a CAGR of 8.1% during 2025-2034. The increasing awareness of Fabry disease and improvements in genetic screening programs have led to more diagnoses, which is driving demand for intravenous therapies. Clinical studies consistently show that intravenous ERT is more effective at stabilizing symptoms over the long term, making it the preferred treatment. However, oral therapies are gaining attention due to their potential convenience and improved patient adherence.
North America generated USD 945.8 million in revenue from the Fabry Disease Treatment Market in 2024, making it a key region for market expansion. Growth is driven by favorable government initiatives, rising incidence rates, and the presence of leading healthcare companies specializing in rare disease treatments. The increasing number of treatment procedures in the U.S., coupled with high healthcare expenditure and strong reimbursement policies, has significantly contributed to market growth. The region remains at the forefront of research and development in Fabry disease treatments, with multiple clinical trials and new drug approvals shaping the competitive landscape.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising cases of fabry disease across the globe
- 3.2.1.2 Growing awareness among specialists and physicians
- 3.2.1.3 Advancements in fabry disease treatment therapies
- 3.2.1.4 Rising health awareness and demand for early-stage diagnosis
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High treatment cost
- 3.2.2.2 Limited treatment options
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Future market trends
- 3.5 Pricing analysis
- 3.6 Product pipeline analysis
- 3.7 Regulatory landscape
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Treatment, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Enzyme replacement therapy (ERT)
- 5.3 Chaperone treatment
- 5.4 Other treatment types
Chapter 6 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Intravenous
- 6.3 Oral
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Homecare settings
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Amicus Therapeutics
- 9.2 Avrobio
- 9.3 Freeline Therapeutics
- 9.4 Idorsia Pharmaceuticals
- 9.5 ISU Abxis
- 9.6 JCR Pharmaceuticals
- 9.7 Novartis
- 9.8 Pfizer
- 9.9 Protalix BioTherapeutics
- 9.10 Sanofi
- 9.11 Takeda Pharmaceuticals
- 9.12 Viatris




