PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1684650

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1684650
CAR T-cell Therapy Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
The Global CAR T-Cell Therapy Market, valued at USD 4.3 billion in 2024, is projected to experience a robust growth trajectory, with an impressive CAGR of 30.5% from 2025 to 2034. Chimeric Antigen Receptor (CAR) T-cell therapy is revolutionizing cancer treatment, marking a significant milestone in oncology. This advanced form of immunotherapy involves the genetic modification of a patient's T-cells to better target and eliminate cancer cells. CAR T-cell therapy is not only offering new hope for patients but also changing the way we approach the treatment of certain cancers. With its growing adoption and advancements in technology, this market is poised for continuous expansion.
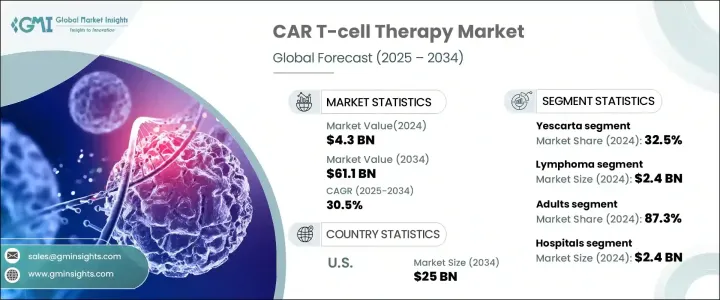
The global market is divided into several key products, including Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, Yescarta, and others. Among these, Yescarta stands out as the market leader, capturing 32.5% of the total market share in 2024. The primary reason for Yescarta's market dominance lies in its proven effectiveness for patients with B-cell lymphomas, particularly in relapsed or refractory cases where traditional treatments often fall short. As more patients seek alternatives for aggressive forms of cancer, the demand for Yescarta continues to grow, further cementing its leadership in the space.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.3 Billion |
| Forecast Value | $61.1 Billion |
| CAGR | 30.5% |
In terms of indications, the CAR T-cell therapy market is categorized into leukemia, lymphoma, multiple myeloma, and others, with lymphoma generating the highest revenue of USD 2.4 billion in 2024. Diffuse large B-cell lymphoma (DLBCL), a common form of non-Hodgkin lymphoma is a major contributor to this growth. DLBCL's high relapse rates following conventional therapies have driven demand for CAR T-cell treatments, which have shown remarkable efficacy in targeting and treating aggressive lymphoma. As a result, CAR T-cell therapies are increasingly viewed as a vital solution in managing these challenging cancer types, offering new hope to patients with few options left.
The U.S. CAR T-cell therapy market is expected to reach USD 25 billion by 2034, with strong growth fueled by supportive regulatory frameworks. The U.S. Food and Drug Administration (FDA) has played a key role in the rapid development and commercialization of CAR T-cell therapies, providing critical regulatory support through expedited approval processes such as Breakthrough Therapy Designations, Orphan Drug status, and Fast Track pathways. These initiatives have fast-tracked the availability of CAR T-cell therapies, ensuring timely access for patients in need of life-saving treatments.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing cases of cancer malignancies across population
- 3.2.1.2 Rising inclination towards targeted treatment
- 3.2.1.3 Robust product pipeline with regulatory approvals across geographies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High drug cost impedes the market growth
- 3.2.2.2 Side-effects of CAR T-cell therapy
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Abecma (idecabtagene vicleucel)
- 5.3 Breyanzi (lisocabtagene maraleucel)
- 5.4 Carvykti (ciltacabtagene autoleucel)
- 5.5 Kymriah (tisagenlecleucel)
- 5.6 Tecartus (brexucabtagene autoleucel)
- 5.7 Yescarta (axicabtagene ciloleucel)
- 5.8 Other products
Chapter 6 Market Estimates and Forecast, By Indication, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Leukemia
- 6.3 Lymphoma
- 6.4 Multiple myeloma
- 6.5 Other indications
Chapter 7 Market Estimates and Forecast, By Demographic, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Adults
- 7.3 Pediatric
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Cancer treatment centers
- 8.4 Specialty clinics
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Allogene Therapeutics
- 10.2 Autolus Therapeutics
- 10.3 bluebird bio
- 10.4 Bristol-Myers Squibb Company
- 10.5 CRISPR Therapeutics
- 10.6 Gilead Sciences
- 10.7 GSK plc.
- 10.8 ImmunoAct
- 10.9 Johnson & Johnson
- 10.10 JW Therapeutics (Shanghai)
- 10.11 Medigene AG
- 10.12 Merck KGaA
- 10.13 Novartis AG
- 10.14 Sangamo Therapeutics
- 10.15 Sorrento Therapeutics




