PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1666711

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1666711
eClinical Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
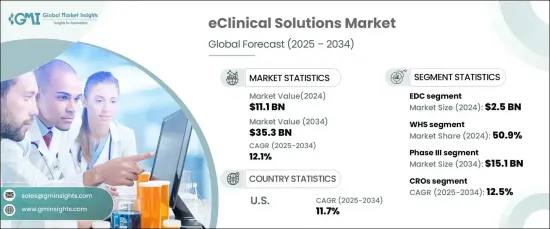
The Global EClinical Solutions Market, valued at USD 11.1 billion in 2024, is forecasted to grow at a CAGR of 12.1% from 2025 to 2034. This rapid growth is driven by the increasing adoption of decentralized clinical trials, which utilize digital technologies to streamline data collection and improve patient engagement. The rising use of wearable devices, mobile health applications, and electronic health records is enhancing real-time monitoring and data accuracy.
Regulatory bodies are also advocating for digital tools to optimize trial efficiency and maintain patient safety standards. Additionally, the escalating volume of clinical trials worldwide, fueled by advancements in personalized medicine and treatments for chronic conditions, is amplifying the need for advanced eClinical solutions. These platforms simplify complex processes such as patient recruitment, compliance monitoring, and real-time data analytics, making them essential in managing the growing intricacies of large-scale trials.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $11.1 Billion |
| Forecast Value | $35.3 Billion |
| CAGR | 12.1% |
eClinical solutions encompass advanced software and digital platforms designed to streamline clinical trial management. These tools integrate data from multiple sources, including electronic data capture (EDC) systems, clinical trial management systems (CTMS), and electronic patient-reported outcomes (ePRO), ensuring improved accuracy and regulatory compliance. The EDC segment led the market in 2024, generating USD 2.5 billion, due to its ability to enhance data processing, minimize manual errors, and accelerate real-time monitoring. As trials become more complex, the demand for such systems continues to rise.
Web-hosted solutions accounted for the largest market share of 50.9% in 2024. Their scalability, affordability, and ease of deployment make them ideal for decentralized trials. These solutions enable remote access, seamless integration with other digital tools, and robust security features, driving their widespread adoption. Their ability to facilitate real-time collaboration and data sharing has further solidified their market position.
The phase III clinical trial segment dominated the market in 2024, projected to reach USD 15.1 billion by 2034. This phase involves large-scale testing to evaluate treatment safety and efficacy, necessitating efficient data management and monitoring. eClinical tools enhance these processes by enabling real-time data capture and ensuring compliance with regulatory requirements.
Contract research organizations (CROs) are set to grow at a 12.5% CAGR over the forecast period. The rising trend of outsourcing clinical research to CROs, driven by their expertise and efficiency, is a key market driver. These organizations utilize eClinical solutions to manage complex multinational trials, streamline operations, and ensure real-time data analysis.
The US dominated the North American market in 2024 and is expected to maintain its lead with a CAGR of 11.7%. This is attributed to the country's robust pharmaceutical and biotechnology industries, as well as its emphasis on technological innovation and decentralized trials. The presence of major research institutions and regulatory bodies further supports the adoption of advanced eClinical technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rise in government funding grants for clinical trials
- 3.2.1.2 Growing demand for outsourcing clinical trials to CROs
- 3.2.1.3 Increasing adoption of software solutions in clinical trials
- 3.2.1.4 Increasing R&D expenditure across the globe
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High implantation cost
- 3.2.2.2 Low adoption and lack of awareness pertaining to eClinical solutions in developing countries
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Software landscape
- 3.6 Porter’s analysis
- 3.7 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Solution, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Randomization and trial supply management
- 5.3 Clinical data management system (CDMS)
- 5.4 Clinical trial management system (CTMS)
- 5.5 Electronic clinical outcome assessment (eCOA)
- 5.6 Electronic trial master file (eTMF)
- 5.7 Electronic data capture (EDC)
- 5.8 Other solutions
Chapter 6 Market Estimates and Forecast, By Delivery Mode, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Licensed enterprise (on-premise) solutions
- 6.3 Cloud-based (SAAS) solution (CBS)
- 6.4 Web-hosted (on-demand) solution (WHS)
Chapter 7 Market Estimates and Forecast, By Clinical Trial Phase, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Phase I
- 7.3 Phase II
- 7.4 Phase III
- 7.5 Phase IV
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Contract research organizations (CROs)
- 8.3 Medical device companies
- 8.4 Pharma/biotech companies
- 8.5 Hospitals and clinics
- 8.6 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Poland
- 9.3.7 Switzerland
- 9.3.8 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.4.6 Indonesia
- 9.4.7 Thailand
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.5.4 Argentina
- 9.5.5 Chile
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
- 9.6.4 Egypt
Chapter 10 Company Profiles
- 10.1 Acralyon
- 10.2 Anju Software (OmniComm System)
- 10.3 Bio-optronics
- 10.4 Clario
- 10.5 Dassault Systemes (Medidata)
- 10.6 Datatrak
- 10.7 Ennov
- 10.8 IBM
- 10.9 Medsharing
- 10.10 Multihealth Group (Clinfile)
- 10.11 OpenClinica
- 10.12 Oracle Corporation
- 10.13 Parexel International
- 10.14 Signant Health (CRF Health)
- 10.15 Veeva Systems




