PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1741035

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1741035
C-Reactive Protein Testing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
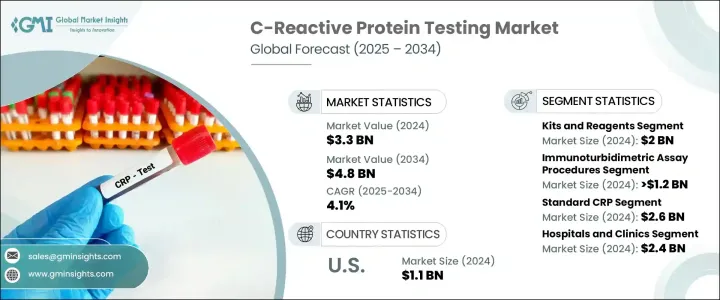
The Global C-Reactive Protein Testing Market was valued at USD 3.3 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 4.8 billion by 2034. The rising prevalence of chronic conditions is one of the key drivers fueling market growth, as healthcare professionals increasingly rely on CRP tests for accurate and timely diagnosis. This growing demand is also supported by the expanding adoption of CRP testing in routine health screenings and disease management protocols. The test measures the concentration of C-reactive protein, which the liver produces in response to inflammation. Its levels can spike due to a variety of triggers, including infections, inflammatory diseases, and cardiovascular conditions. Elevated CRP levels serve as an important marker to detect and monitor inflammation in the body, assisting medical professionals in assessing the severity of a patient's condition and formulating appropriate treatment strategies.
As diagnostic capabilities evolve, CRP testing continues to gain traction in healthcare systems worldwide. With its ability to deliver rapid results, the test is becoming indispensable for early intervention, especially in clinical settings where time is critical. CRP testing has also become integral in tracking disease progression and therapeutic effectiveness. The increased use of these tests has not only improved patient outcomes but has also contributed to cost-effective care delivery, making them a reliable choice for both small clinics and large healthcare facilities. The market's growth is further supported by advancements in test accuracy, efficiency, and ease of use, which have enhanced the utility of CRP testing across various medical specialties. As a result, CRP testing is now widely used as a diagnostic tool in managing conditions ranging from infections and autoimmune diseases to inflammatory and cardiovascular disorders.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.3 Billion |
| Forecast Value | $4.8 Billion |
| CAGR | 4.1% |
In terms of products, the market is segmented into instruments and kits & reagents. In 2024, the kits and reagents segment led the market with revenue totaling USD 2 billion. These products are widely adopted due to their compatibility with diverse testing platforms, allowing their application across multiple assay techniques. Their versatility and ability to produce fast results make them suitable for various clinical settings, where timely decisions are essential. The ease of integration and efficiency of these kits further drive their preference over more complex testing procedures.
Based on assay type, the market includes enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CLIA), immunoturbidimetric assay, and others. Immunoturbidimetric assays accounted for the highest market share in 2024, reaching a value of over USD 1.2 billion. These assays are favored for their cost-effectiveness and quick turnaround times, making them an accessible choice for smaller healthcare providers and laboratories with limited resources. Their ability to deliver reliable results swiftly enhances their role in clinical practice, where fast diagnostics are essential for effective patient management.
By detection range, the market is divided into high-sensitivity CRP (Hs-CRP) and standard CRP. The standard CRP segment dominated in 2024 and is expected to reach USD 2.6 billion by 2034. The widespread use of standard CRP tests in hospitals and diagnostic centers for general inflammation detection has contributed to their market lead. Their proven clinical reliability and affordability further drive their continued use.
In terms of application, the market includes cardiovascular diseases, infectious diseases, chronic inflammatory diseases, and other uses. The cardiovascular diseases segment generated over USD 1.2 billion in revenue in 2024. CRP testing is a common component in cardiovascular risk assessment, aiding healthcare professionals in monitoring disease progression and evaluating treatment efficacy. The growing emphasis on preventive care and early intervention in heart-related conditions supports its consistent demand in this application area.
Regarding end use, the market is segmented into hospitals and clinics, diagnostic laboratories, and other end users. Hospitals and clinics were the largest end-use segment in 2024, with revenue reaching USD 2.4 billion. These facilities regularly use CRP tests for patients presenting with symptoms of inflammation, infections, or cardiovascular events. The ability to quickly process CRP test results is a key advantage in emergency and primary care settings, where immediate clinical decisions are critical. The broad scope of departments utilizing CRP testing-from internal medicine to infectious disease units-adds to its high utilization rate.
In the United States, the CRP testing market was valued at USD 1.1 billion in 2024 and is expected to grow at a CAGR of 3.2% through the forecast period. The shift toward preventive healthcare, supported by policies that encourage early diagnosis, is boosting the adoption of CRP testing across the country. Growing awareness around inflammatory conditions and better health education are also contributing to increased test usage among both healthcare providers and patients.
Approximately 55% of the market is controlled by five leading companies, including major players that continue to innovate and launch advanced testing solutions. These companies are focusing on developing cost-effective, user-friendly, and high-precision CRP testing products that meet the rising demand from both developed and developing healthcare markets. Their strategic emphasis on technological innovation and streamlined diagnostic solutions is intensifying competition and pushing market growth forward.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chronic disorders such as inflammatory diseases
- 3.2.1.2 Surge in demand for CRP testing for diagnosis and management of chronic diseases
- 3.2.1.3 Advances in technology
- 3.2.1.4 Growing demand for direct-to-home testing services
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of standardization
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the Industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Reimbursement scenario
- 3.7 Technology landscape
- 3.8 Future market trends
- 3.9 Porter's analysis
- 3.10 GAP analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Company market share analysis
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Instruments
- 5.3 Kits and reagents
Chapter 6 Market Estimates and Forecast, By Assay Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Chemiluminescence immunoassay (CLIA)
- 6.3 Enzyme-linked immunosorbent assay (ELISA)
- 6.4 Immunoturbidimetric assay
- 6.5 Other assay types
Chapter 7 Market Estimates and Forecast, By Detection Range, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 High-sensitivity CRP (hs-CRP)
- 7.3 Standard CRP
Chapter 8 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Cardiovascular diseases
- 8.3 Infectious diseases
- 8.4 Chronic inflammatory diseases
- 8.5 Other applications
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals and clinics
- 9.3 Diagnostic laboratories
- 9.4 Other end uses
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Abbott
- 11.2 Agilent
- 11.3 AIDIAN
- 11.4 Boditech
- 11.5 Creative Diagnostics
- 11.6 CTK BIOTECH
- 11.7 Danaher
- 11.8 DxGen Corp
- 11.9 Getein Biotech
- 11.10 GOLDSITE
- 11.11 HORIBA
- 11.12 Labcorp
- 11.13 Merck
- 11.14 OptiBio
- 11.15 RANDOX
- 11.16 Roche
- 11.17 SIEMENS Healthineers




