PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766337

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766337
Disposable Endoscopes Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
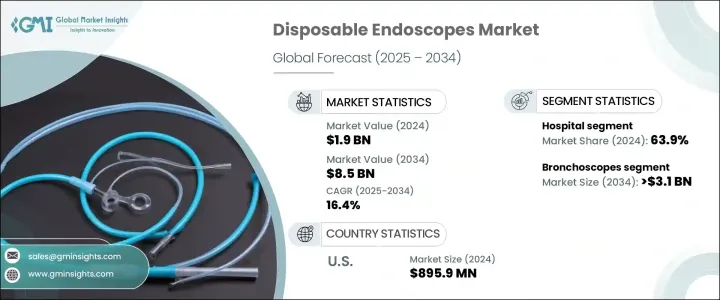
The Global Disposable Endoscopes Market was valued at USD 1.9 billion in 2024 and is estimated to grow at a CAGR of 16.4% to reach USD 8.5 billion by 2034. This notable growth is largely driven by the increasing preference for minimally invasive procedures and the growing prevalence of chronic diseases such as cancer and gastrointestinal disorders. A steady rise in conditions like inflammatory bowel disease, peptic ulcers, colorectal cancer, and esophageal complications-largely attributed to poor dietary habits, sedentary lifestyles, and aging populations-continues to boost the need for early diagnostic tools. As these conditions often demand frequent examinations, disposable endoscopes have emerged as a safer option, reducing the risk of hospital-acquired infections and cross-contamination that may occur with reusable scopes.
Designed for single-use, disposable endoscopes-whether flexible or rigid-offer both diagnostic and therapeutic capabilities. One of their major advantages is that they eliminate the need for complex sterilization processes, making them ideal for use in point-of-care or emergency settings. The introduction of cost-effective designs integrated with CMOS sensor technology has improved imaging clarity and real-time visibility during procedures, driving further adoption in various healthcare environments, especially in resource-limited settings.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.9 Billion |
| Forecast Value | $8.5 Billion |
| CAGR | 16.4% |
The bronchoscopes segment is forecasted to witness strong expansion, growing at a CAGR of 17% to reach USD 3.1 billion by 2034. The rising inclination toward single-use bronchoscopes stems from their ability to minimize infection risks in high-stakes environments like ICUs. These devices are now preferred for their ready-to-use functionality, especially in critical care and trauma scenarios where rapid intervention is necessary and time-consuming reprocessing of traditional scopes is impractical.
In 2024, hospitals accounted for the largest share of the market, generating 63.9%. With a high procedural load and established infrastructure, hospitals are increasingly transitioning to disposable endoscopic solutions to enhance safety and operational efficiency. Traditional reusable scopes, especially in surgical units, present a greater risk of infection transmission. In contrast, disposable scopes offer a lower infection profile, making them a preferred choice in critical departments where HAIs pose a significant risk to patient health and institutional costs.
United States Disposable Endoscopes Market generated USD 895.9 million in 2024 and is set to grow at a CAGR of 15.3% through 2034. The country's leading position is supported by a well-established healthcare system, high surgical volumes, and early adoption of innovative technologies. Growing emphasis on infection control practices has further fueled demand for disposable solutions. Favorable reimbursement structures, regulatory support from the FDA, and substantial R&D investments from domestic companies have also created an environment conducive to rapid product development and market expansion.
Key players leading the Disposable Endoscopes Industry include Olympus, HugeMed, Boston Scientific, RICHARD WOLF, Flexicare, Ambu, Karl Storz, CooperSurgical, Parburch MEDICAL, and Hoya Corporation. Leading companies in this market are focusing on new product launches, strategic collaborations, and technological innovation to strengthen their presence. Many are investing in advanced imaging technologies and ergonomics to enhance usability and procedural efficiency. Mergers and acquisitions are also being pursued to expand product portfolios and gain access to untapped regional markets. Some players are emphasizing scalable production capabilities and affordable designs to cater to growing demand from emerging economies. At the same time, partnerships with hospitals and clinical research institutions are helping to validate product performance and drive broader adoption across healthcare settings.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Product type
- 2.2.3 End use
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising incidences of gastrointestinal disorders, cancer, and other chronic conditions
- 3.2.1.2 Increasing demand for minimally invasive procedures
- 3.2.1.3 Technological advancements in disposable endoscopes
- 3.2.1.4 Increased focus on contamination and infection control
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of skilled physicians and endoscopists
- 3.2.3 Market opportunities
- 3.2.3.1 Regulatory push for infection control
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 RoW
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Pricing analysis
- 3.7 Reimbursement scenario
- 3.8 Gap analysis
- 3.9 Porter's analysis
- 3.10 Pipeline analysis
- 3.11 PESTEL analysis
- 3.12 Gap analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 By region
- 4.3.1.1 North America
- 4.3.1.2 Europe
- 4.3.1.3 RoW
- 4.3.1 By region
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn, Units)
- 5.1 Key trends
- 5.2 Bronchoscopes
- 5.3 Cystoscopes
- 5.4 Duodenoscopes
- 5.5 Hysteroscopes
- 5.6 Laryngoscopes
- 5.7 Ureteroscopes
- 5.8 Other product types
Chapter 6 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Hospitals
- 6.3 Ambulatory surgical centers
- 6.4 Other end use
Chapter 7 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn, Units)
- 7.1 Key trends
- 7.2 North America
- 7.2.1 U.S.
- 7.2.2 Canada
- 7.3 Europe
- 7.3.1 Germany
- 7.3.2 UK
- 7.3.3 France
- 7.3.4 Spain
- 7.3.5 Italy
- 7.3.6 Netherlands
- 7.4 RoW
Chapter 8 Company Profiles
- 8.1 Ambu
- 8.2 Boston Scientific
- 8.3 CooperSurgical
- 8.4 Flexicare
- 8.5 Hoya Corporation
- 8.6 HugeMed
- 8.7 Karl Storz
- 8.8 OLYMPUS
- 8.9 Parburch MEDICAL
- 8.10 RICHARD WOLF




