PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871286

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871286
Real World Evidence Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
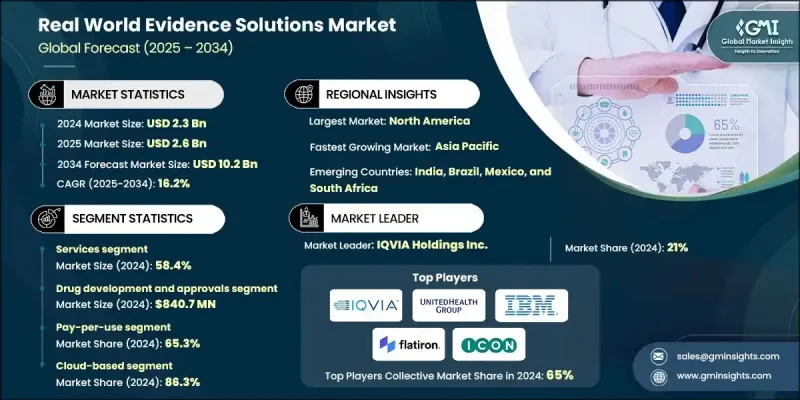
The Global Real World Evidence Solutions Market was valued at USD 2.3 billion in 2024 and is estimated to grow at a CAGR of 16.2 % to reach USD 10.2 billion by 2034.
Market growth is driven by the rising need for real-time clinical insights, the growing demand for cost-effective drug development, and the expanding use of big data analytics in healthcare. RWE solutions are transforming pharmaceutical research, helping companies analyze patient outcomes, treatment efficacy, and safety using real-world data from electronic health records, insurance databases, and patient registries. The growing emphasis on evidence-based decision-making by healthcare providers, regulators, and payers is further fueling market growth. Moreover, advancements in AI and machine learning are enhancing the accuracy and predictive capabilities of RWE platforms, enabling faster clinical trial design and post-market surveillance.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.3 Billion |
| Forecast Value | $10.2 Billion |
| CAGR | 16.2% |
The market is primarily segmented by component, with the services segment held 58.4% share in 2024. This dominance is attributed to the increasing outsourcing of data management, analytics, and regulatory reporting to specialized service providers. The services segment plays a crucial role in assisting biopharma and medical device firms in real-world data integration, interpretation, and compliance management, reducing time-to-market and research costs.
In terms of end-use, the pharmaceutical and medical devices companies segment held 60.4% in 2024, driven by the growing need to demonstrate drug effectiveness and safety beyond clinical trial environments. These companies are leveraging RWE solutions for label expansion, lifecycle management, and strategic decision-making to improve patient outcomes and regulatory compliance.
North America Real World Evidence Solutions Market held 43.2% share in 2024, owing to the region's advanced healthcare infrastructure, supportive regulatory frameworks, and widespread adoption of digital health technologies. The U.S. leads the region due to the presence of major pharmaceutical companies, strong government initiatives promoting RWE-based clinical research, and extensive use of electronic health records and claims databases. The region's focus on precision medicine and patient-centric care continues to drive RWE adoption across healthcare ecosystems.
Key players operating in the Global Real World Evidence Solutions Market include IQVIA, Syneos Health, Oracle Corporation, Flatiron Health, ICON plc, and Medidata Solutions. Companies in the Real World Evidence Solutions Market are strengthening their presence through strategic partnerships, data integration capabilities, and AI-driven analytics innovations. Many are expanding collaborations with healthcare providers and payers to enhance data accessibility and improve real-world study quality. Key players are also investing in scalable cloud-based platforms to streamline data capture, storage, and interpretation while ensuring compliance with evolving data privacy regulations. Additionally, mergers and acquisitions are being leveraged to expand service portfolios and geographic reach.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Component trends
- 2.2.3 Application trends
- 2.2.4 Revenue model trends
- 2.2.5 Deployment trends
- 2.2.6 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing focus towards accelerating drug development and cost reduction
- 3.2.1.2 Growing demand for real-time safety and efficacy monitoring of drugs and medical devices
- 3.2.1.3 Increasing adoption of RWE Solutions for informed reimbursement decision-making
- 3.2.1.4 Increasing adoption of data analytics services in clinical decision making.
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of standardization in integration and interoperability of real-world data
- 3.2.2.2 Shortage of skilled professionals
- 3.2.3 Market opportunities
- 3.2.3.1 Emerging therapeutic areas expansion beyond oncology
- 3.2.3.2 Focus on patient-generated health data integration
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
- 4.7 Key developments
- 4.7.1 Mergers and acquisitions
- 4.7.2 Partnerships and collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Component, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Services
- 5.2.1 Data collection and integration
- 5.2.2 Study design and execution
- 5.2.2.1 Prospective observational studies
- 5.2.2.2 Retrospective database studies
- 5.2.2.3 Site-centric studies
- 5.2.2.4 Registry-based studies
- 5.2.2.5 Hybrid studies
- 5.2.3 Regulatory and market access support
- 5.2.4 Evidence network
- 5.2.5 Other services
- 5.3 Data sets
- 5.3.1 Disparate data sets
- 5.3.1.1 Clinical settings data
- 5.3.1.2 Claims data sets
- 5.3.1.3 Pharmacy data sets
- 5.3.1.4 Patient powered data sets
- 5.3.1.5 Registry-based data sets
- 5.3.1 Disparate data sets
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Drug development and approval
- 6.2.1 Oncology
- 6.2.2 Cardiovascular disease
- 6.2.3 Neurology
- 6.2.4 Immunology
- 6.2.5 Other therapeutic areas
- 6.3 Medical device development and approvals
- 6.4 Post-market surveillance
- 6.5 Market access and reimbursement/coverage decision-making
Chapter 7 Market Estimates and Forecast, By Revenue Model, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pay-per-use (value-based pricing)
- 7.3 Subscription
Chapter 8 Market Estimates and Forecast, By Deployment Model, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 On-premise
- 8.3 Cloud-based
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Pharmaceutical and medical device companies
- 9.3 Healthcare payers
- 9.4 Healthcare providers
- 9.5 Other End use
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 1.1.1 U.S.
- 1.1.2 Canada
- 10.3 Europe
- 1.1.3 Germany
- 1.1.4 UK
- 1.1.5 France
- 1.1.6 Spain
- 1.1.7 Italy
- 1.1.8 Netherlands
- 10.4 Asia Pacific
- 1.1.9 China
- 1.1.10 Japan
- 1.1.11 India
- 1.1.12 Australia
- 1.1.13 South Korea
- 10.5 Latin America
- 1.1.14 Brazil
- 1.1.15 Mexico
- 1.1.16 Argentina
- 10.6 Middle East and Africa
- 1.1.17 South Africa
- 1.1.18 Saudi Arabia
- 1.1.19 UAE
Chapter 11 Company Profiles
- 11.1 Aetion, Inc.
- 11.2 Cytel Inc.
- 11.3 Flatiron Health Inc.
- 11.4 Fortrea Inc.
- 11.5 ICON plc
- 11.6 IBM Corporation
- 11.7 IQVIA Holdings Inc.
- 11.8 Medidata Solutions
- 11.9 Merative
- 11.10 Optum, Inc.
- 11.11 Oracle Corporation
- 11.12 Parexel International Corporation
- 11.13 Syneos Health Inc.
- 11.14 Tempus Labs Inc.
- 11.15 Thermo Fisher Scientific Inc.
- 11.16 TriNetX LLC.




