PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871275

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871275
Bioanalytical Testing Services Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
The Global Bioanalytical Testing Services Market was valued at USD 4.2 billion in 2024 and is estimated to grow at a CAGR of 8.2% to reach USD 9.3 billion by 2034.
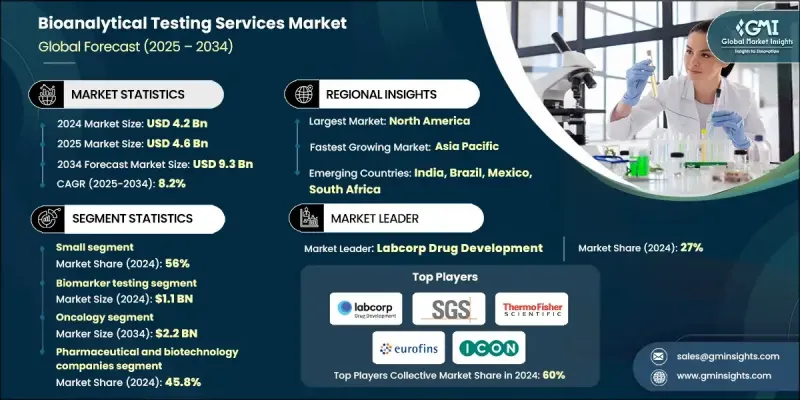
The steady growth is fueled by the increasing trend of outsourcing laboratory testing, rapid advancements in bioanalytical technologies, and the rising volume of drug development and approval processes. The surge in clinical trials and research activities also supports market expansion. Bioanalytical testing services involve analyzing biological samples, including blood, tissue, and urine, to quantify drugs, metabolites, biomarkers, and other analytes in biological matrices. These services are crucial for the pharmaceutical and biotechnology industries to conduct pharmacokinetic studies, bioequivalence evaluations, method validations, and clinical trial support. They ensure drug safety, efficacy, and regulatory compliance while providing essential data for pharmacodynamic studies and biomarker analysis. Growing demand for precise, reliable, and high-quality bioanalytical data continues to drive the adoption of these services globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.2 Billion |
| Forecast Value | $9.3 Billion |
| CAGR | 8.2% |
The small molecule segment held a 56% share in 2024. The increasing demand for small molecule testing is driven by the continued prevalence of small molecule drugs in various therapeutic areas. Their relatively simpler chemical structures, established regulatory pathways, and cost-effective production make them favorable for pharmaceutical companies. Moreover, the surge in generic drug approvals and patent expirations for branded small-molecule drugs has escalated the need for bioequivalence studies.
The pharmaceutical and biotechnology companies segment held a 45.8% share in 2024. These organizations rely heavily on bioanalytical testing to support the development of complex biologics, biosimilars, and targeted therapies, ensuring regulatory compliance and robust data integrity. Collaborations between bioanalytical service providers and pharmaceutical or biotech companies are expanding rapidly, enhancing operational efficiency, accelerating drug development timelines, and maintaining high-quality testing standards.
United States Bioanalytical Testing Services Market was valued at USD 1.5 billion in 2024. Growth is driven by increased R&D investments by pharmaceutical and biotechnology firms, particularly in biologics development, and strategic partnerships with contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs). North America's bioanalytical testing services market benefits from advanced pharmaceutical and biotechnology infrastructure, extensive research activities, and a high number of drug discovery and development projects requiring specialized testing services.
Key companies in the Global Bioanalytical Testing Services Market include Thermo Fisher Scientific Inc., Intertek Group Plc, WuXi AppTec, ICON Plc, Pace Analytical Services LLC, BioAgilytix, Eurofins Scientific, Labcorp Drug Development, Charles River Laboratories, Inc., SGS Societe Generale de Surveillance SA, Frontage Labs, Syneos Health, Cambrex Corporation, Altasciences, and LGC Limited. Companies in the Global Bioanalytical Testing Services Market are strengthening their presence through several strategic approaches. They are investing heavily in research and development to enhance analytical capabilities, improve sensitivity, and expand service portfolios. Strategic collaborations and partnerships with pharmaceutical, biotechnology, and contract research organizations accelerate market reach and improve service efficiency. Mergers and acquisitions help consolidate technological expertise, expand geographical presence, and scale operations. Firms are also focusing on regulatory approvals, accreditation, and method validation to enhance credibility and attract high-profile clients.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Molecule type trends
- 2.2.3 Test type trend
- 2.2.4 Application trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increased trend of outsourcing laboratory testing services
- 3.2.1.2 Rising advancements in bioanalytical technology
- 3.2.1.3 Growing drug development and approval processes
- 3.2.1.4 Increasing volume of ongoing research activities and clinical trials
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Complex regulatory framework for maintaining laboratories
- 3.2.2.2 High cost of advanced analytical instruments and technologies
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of clinical trials in emerging markets
- 3.2.3.2 Integration of AI and automation in bioanalytical workflows
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2024
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Molecule Type, 2021 - 2034 ($ Bn)
- 5.1 Key trends
- 5.2 Small molecule
- 5.3 Large molecule
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Bn)
- 6.1 Key trends
- 6.2 DMPK Testing
- 6.3 Biomarker testing
- 6.4 Virology testing
- 6.4.1 In-vivo virology testing
- 6.4.2 In-vitro virology testing
- 6.5 Serology testing
- 6.6 Immunogenicity testing
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Bn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Neurology
- 7.4 Infectious diseases
- 7.5 Gastroenterology
- 7.6 Cardiology
- 7.7 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Bn)
- 8.1 Key trends
- 8.2 Pharmaceutical and biotechnology companies
- 8.3 Contract research organizations
- 8.4 Research and academic institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Bn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Altasciences
- 10.2 BioAgilytix
- 10.3 Cambrex Corporation
- 10.4 Charles River Laboratories, Inc.
- 10.5 Eurofins Scientific
- 10.6 Frontage Labs
- 10.7 ICON Plc
- 10.8 Intertek Group Plc
- 10.9 Labcorp Drug Development
- 10.10 LGC Limited
- 10.11 Pace Analytical Services LLC
- 10.12 SGS Societe Generale de Surveillance SA
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific Inc.
- 10.15 WuXi AppTec




