PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1708300

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1708300
Global Blarcamesine Market, By Route of Adminsitration, By Application, By Distribution Channel, By Geography .
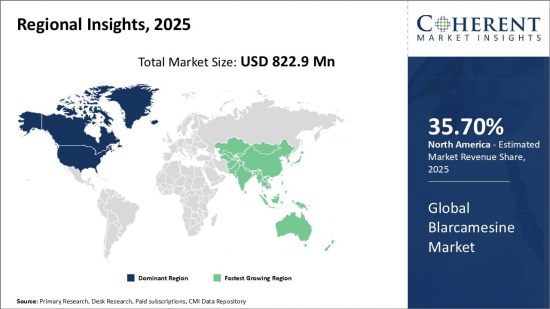
Global Blarcamesine Market is estimated to be valued at USD 822.9 Mn in 2025 and is expected to reach USD 1,082.8 Mn by 2032, growing at a compound annual growth rate (CAGR) of 4.0% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 822.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.00% | 2032 Value Projection: | USD 1,082.8 Mn |
Blarcamesine is an agonist of the intracellular sigma-1 chaperone protein. Specifically, it is a mixed ligand for sigma1/muscarinic receptors. Expressed in most tissues and located at focal contacts between mitochondria and the endoplasmic reticulum, the sigma-1 receptor forms heterodimers with many other membrane receptors and, as such, influences multiple cellular pathways and physiological processes. Blarcamesine reportedly binds the sigma-1 receptor in the high nanomolar and the muscarinic receptor in the low micromolar range. The compound has been reported to have memory-preserving and neuroprotective effects in mice treated with the muscarinic receptor antagonist scopolamine, with synthetic AB oligomer injection, or with the NMDA receptor agonist dizocilpine (Villard et al., 2011). Other studies in the AB oligomer injection model suggest that blarcamesine may block tau hyperphosphorylation and protect mitochondria.
Market Dynamics:
Increasing government approval is expected to drive the growth of the market over the forecast period. For instance, on December 19, 2024, Anavex Life Sciences, a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders, announced that the Committee for Medicinal Products for Human Use (CHMP) within the European Medicines Agency (EMA) agreed that oral blarcamesine for Alzheimer's disease is eligible for submission of an application for a Union Marketing Authorization in the EU under the European Medicines Agency's centralized procedure.
Key features of the study:
- This report provides in-depth analysis of the global blarcamesine market, and provides market size (US$ Mn) and compound annual growth rate (CAGR %) for the forecast period (2026-2032), considering 2024 as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global blarcamesine market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
- Key companies covered as a part of this study include Anavex Life Sciences
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- Global blarcamesine market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global blarcamesine market
Detailed Segmentation:
- Global Blarcamesine Market, By Route of Administration
- Oral
- Parentral
- Global Blarcamesine Market, By Application
- Alzheimer's Disease
- Parkinson's Disease
- Dementia
- Rett Syndrome
- Others
- Global Blarcamesine Market, By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Global Blarcamesine Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles
- Anavex Life Sciences
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Blarcamesine Market, By Route of Administration
- Global Blarcamesine Market, By Application
- Global Blarcamesine Market, By Distribution Channel
- Global Blarcamesine Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Increasing research and development activities
- Strict regulatory requirements
- Emerging markets
- Key Highlights
- Regulatory Scenario
- Recent Trends
- PEST Analysis
- PORTER's Analysis
- Mergers, Acquisitions, and Collaborations
4. Global Blarcamesine Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global Blarcamesine Market, By Route of Administration, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032(%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Oral
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Parenteral
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
6. Global Blarcamesine Market, By Application, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032(%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Alzheimer's Disease
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Parkinson's Disease
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Dementia
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Rett Syndrome
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
7. Global Blarcamesine Market, By , 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032(%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
8. Global Blarcamesine Market, By Region, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032(%)
- Y-o-Y Growth Analysis, For Region,
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Route of Administration, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020-2032,(US$ Mn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Company Profile
- Anavex Life Sciences
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
10. Section
- References
- Research Methodology
- About us




