PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705841

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705841
Global Angiography Equipment Market, By Product Type, By Technology, By Procedure, By Application, By End User, By Geography
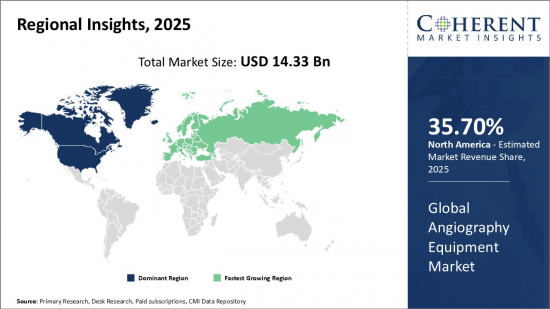
Global Angiography Equipment Market is estimated to be valued at USD 14.33 Bn in 2025 and is expected to reach USD 21.13 Bn by 2032, growing at a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 14.33 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.70% | 2032 Value Projection: | USD 21.13 Bn |
Angiography equipment is commonly used in hospitals and diagnostic centers for minimally invasive surgeries. It helps doctors visualize the insides of blood vessels and organs with high precision. The growing prevalence of cardiovascular diseases and increasing investments in healthcare infrastructure are major factors fueling the demand for angiography equipment. Advancements in imaging technologies like flat panel detectors are enabling manufacturers to develop new generation systems with better image quality and precision. Moreover, rising healthcare expenditure per capita in developing countries creates a conducive environment for market growth over the coming years.
Market Dynamics:
The global angiography equipment market is driven by the rising geriatric population susceptible to conditions like arteriosclerosis and coronary artery disease. Cardiovascular diseases have emerged as a leading cause of mortality worldwide, which is increasing the need for angiography procedures. However, market expansion is negatively impacted by the high installation and maintenance costs of these systems. Stringent regulatory approvals also delay new product launches. On the positive side, manufacturers are continuously innovating to develop low-cost angiography devices suitable for hospitals and clinics in price-sensitive markets. Growing medical tourism in Asia Pacific provides new opportunities for market participants.
On the other hand, market players are focused on adopting various business development strategies, such as product approval from the U.S. Food and Drug Administration and others, which in turn is expected to fuel market growth over the forecast period. For instance, in July 2022, Siemens Healthineers, a global medical device company, announced U.S. Food and Drug Administration (FDA) clearance of the ARTIS icono ceiling, a ceiling-mounted angiography system designed for a wide range of routine and advanced procedures in interventional radiology (IR) and cardiology. The system's high degree of mechanical flexibility also makes it suitable for many surgical procedures.
Key features of the study:
- This report provides in-depth analysis of the global angiography equipment market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global angiography equipment market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
- Key companies covered as a part of this study include B. Braun Melsungen AG, Koninklijke Philips N.V., GE Healthcare, Cardinal Health, Siemens Healthcare Gmbh, Shimadzu Corporation, Medtronic plc, Boston Scientific Corporation, ANGIODYNAMICS, Abbot, Microport Scientific Corporation, Terumo Corporation, CURATIA MEDICAL INC., Cook Medical, Merit Medical Systems, Inc. and Applied Medical Resources Corp
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- The global angiography equipment market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global angiography equipment market
Detailed Segmentation:
- Global Angiography Equipment Market, By Product Type
- Angiography Systems
- Catheters
- Guidewire
- Balloons
- Contrast Media
- Vascular Closure Devices
- Others
- Global Angiography Equipment Market, By Technology
- X-Ray
- MRA
- CT
- Global Angiography Equipment Market, By Procedure
- Coronary
- Endovascular
- Neurovascular
- Global Angiography Equipment Market, By Application
- Diagnostic
- Therapeutic
- Global Angiography Equipment Market, By End User
- Hospitals
- Speciality Clinics
- Ambulatory Surgical Centers
- Diagnostic Centers
- Others
- Global Angiography Equipment Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles
- B.Braun Melsungen AG
- Koninklijke Philips N.V.
- GE Healthcare
- Cardinal Health
- Siemens Healthcare Gmbh
- Shimadzu Corporation
- Medtronic plc
- Boston Scientific Corporation
- ANGIODYNAMICS
- Abbot
- Microport Scientific Corporation
- Terumo Corporation
- CURATIA MEDICAL INC.
- Cook Medical
- Merit Medical Systems, Inc.
- Applied Medical Resources Corp
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Angiography Equipment Market, By Product Type
- Global Angiography Equipment Market, By Technology
- Global Angiography Equipment Market, By Procedure
- Global Angiography Equipment Market, By Application
- Global Angiography Equipment Market, By End User
- Global Angiography Equipment Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Rising prevalence of cardiovascular diseases
- High cost of angiography equipment
- Growing healthcare expenditure
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Service offering Portfolio
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
4. Global Angiography Equipment Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global Angiography Equipment Market, By Product Type, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Angiography Systems
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Catheters
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Guidewire
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Balloons
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Contrast Media
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Vascular Closure Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
6. Global Angiography Equipment Market, By Technology, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- X-Ray
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- MRA
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- CT
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
7. Global Angiography Equipment Market, By Procedure, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Coronary
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Endovascular
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Neurovascular
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
8. Global Angiography Equipment Market, By Application, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Diagnostic
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Therapeutic
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
9. Global Angiography Equipment Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Speciality Clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Ambulatory Surgical Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Diagnostic Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
10. Global Angiography Equipment Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Country, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, For Country 2021 -2032
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Technology, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Procedure, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020-2032,(US$ Bn)
- North Africa
- Central Africa
- South Africa
11. Competitive Landscape
- Braun Melsungen AG
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Koninklijke Philips N.V.
- GE Healthcare
- Cardinal Health
- Siemens Healthcare Gmbh
- Shimadzu Corporation
- Medtronic plc
- Boston Scientific Corporation
- ANGIODYNAMICS
- Abbot
- Microport Scientific Corporation
- Terumo Corporation
- CURATIA MEDICAL INC.
- Cook Medical
- MidWest Homes For Pets
- Merit Medical Systems, Inc.
- Applied Medical Resources Corp
- Analyst Views
12. Section
- Research Methodology
- About us




