PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705635

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705635
Bioburden Testing Market, By Product, By Test Type, By Application, By End User, By Geography
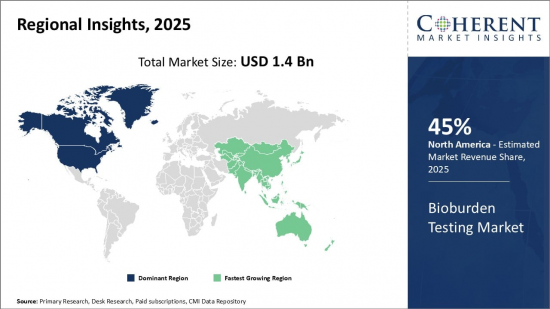
Global Bioburden Testing Market is estimated to be valued at USD 1.4 Bn in 2025 and is expected to reach USD 2.63 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.4 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.40% | 2032 Value Projection: | USD 2.63 Bn |
Bioburden testing refers to the detection and quantification of microorganisms present in or on a product, raw material, or surface. Bioburden testing helps monitor the quality of pharmaceutical and healthcare products by ensuring materials and equipment used in production meet appropriate hygienic standards. It plays a key role in maintaining sterility and preventing contamination of sterile devices, equipment, and finished products. The bioburden testing market is growing due to stringent regulations mandating testing of medical devices, rising penetration of contract manufacturing organizations, and increasing outsourcing of pharmaceutical manufacturing. As medical devices and pharmaceuticals are intended to interact directly with patients, maintaining sterility and preventing microbial growth are critical. Bioburden testing provides quality control measures to validate sterilization processes and detect any contaminants that may have been introduced during manufacturing or shipping. The results of these tests help determine appropriate shelf life and assist in investigations should any issues arise with a product batch in the future.
Market Dynamics:
The global bioburden testing market is driven by stringent regulatory mandates for the testing of bioburden in pharmaceutical and medical device manufacturing. Regulatory agencies like the Food and Drug Administration. FDA and European Medicines Agency EMA require bioburden testing at various stages to ensure the safety and efficacy of non-sterile products. The growth in pharmaceutical and medical device industries has also fueled the demand for bioburden testing. However, the high costs associated with bioburden testing equipment and services limit the growth of this market. The emergence of rapid automated bioburden testing solutions presents lucrative opportunities, as they enable faster results and higher throughput. The adoption of continuous monitoring systems for bioburden testing during manufacturing further offers opportunities in the coming years.
Key features of the study:
- This report provides in-depth analysis of the global bioburden testing market, and provides market size (US$ Billion) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global bioburden testing market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
- Key companies covered as a part of this study include Charles River Laboratories, Merck KGaA, SGS, WuXi AppTec, and Becton, Dickinson and Company ,North American Science Associates Inc. , Nelson Laboratories LLC , Biomerieux SA ,Thermo Fisher ScientifiC and Pacific Biolabs.
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- The global bioburden testing market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts.
Detailed Segmentation:
- Bioburden Testing Market ,By Product
- Consumables
- Culture Media
- Reagents and Kits
- Instruments
- Automated Microbial Identification Systems
- PCR Instruments
- Bioburden Testing Market,By Test Type
- Aerobic Count Testing
- Anaerobic Count Testing
- Fungi/Mold Count Testing
- Spore Count Testing
- Bioburden Testing Market, By Application
- Raw Material Testing
- Medical Device Testing
- In-Process Material Testing
- Sterilization Validation Testing
- Equipment Cleaning Validation
- Bioburden Testing Market, By End Use
- Pharmaceutical & Biotechnology Companies
- Medical Device Manufacturers
- Contract Manufacturing Organizations
- Manufacturers of Food & Beverage and Agricultural Products
- Microbial Testing Laboratories
- Bioburden Testing Market, By Region
- North America
- Latin America
- Europe
- Asia Pacific
- Middle East and Africa
- Company Profiles
- Charles River Laboratories International Inc.
- SGS SA
- Merck KGaA
- Becton
- Dickinson and Company
- Wuxi Apptec
- North American Science Associates Inc.
- Nelson Laboratories LLC
- Biomerieux SA,
- Thermo Fisher Scientific
- Pacific Biolabs.
Table of Content:
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Overview
- Report Description
- Market Definition and Scope
- Executive Summary
- Bioburden Testing Market, By Product
- Bioburden Testing Market, By Test Type
- Bioburden Testing Market, By Application
- Bioburden Testing Market, By End Use
- Bioburden Testing Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Oppertunities
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
4. Bioburden Testing Market Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Impact Bioburden Testing Market
5. Bioburden Testing Market, By Product, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Consumables, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Instruments, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
6. Bioburden Testing Market, By Test Type, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Aerobic Count Testing 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Anaerobic Count Testing 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Fungi/Mold Count Testing 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Spore Count Testing 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
7. Bioburden Testing Market, By Application, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Raw Material Testing , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Medical Device Testing, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- In-process Material Testing , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Sterilization Validation Testing, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecas and Y-o-Y Growth t, 2020 - 2032, ( US$ Billion)
- Equipment Cleaning Validation, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
8. Bioburden Testing Market, By End Use, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Pharmaceutical & Biotechnology Companies , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Medical Device Manufacturers , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth 2020 - 2032, ( US$ Billion)
- Contract Manufacturing Organizations , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Manufacturers Of Food & Beverage And Agricultural Products , 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Microbial Testing Laboratories, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
9. Bioburden Testing Market, By Region, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Share Analysis, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- By Region
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- North America
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Application, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By End Use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Country, 2020 - 2032, ( US$ Billion)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Application, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By End Use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecas and Y-o-Y Growth t, By Country, 2020 - 2032, ( US$ Billion)
- U.K
- German
- Italy
- France
- Spain
- Russia
- Rest Of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Application, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By End Use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Country, 2020 - 2032, ( US$ Billion)
- China
- India
- Japan
- Australia
- South Korea
- Asean
- Rest Of Asia Pacific
- Middle East & Africa
- Introduction
- Market Size and Forecast, By Product and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By Application and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By End Use and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By Country and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- GCC
- Israel
- Rest Of Middle East
- Latin America
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Application, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By End Use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Country, 2020 - 2032, ( US$ Billion)
- Brazil
- Mexico
- Argentina
10. Company Profiles - Bioburden Testing Market
- Charles River Laboratories International, Inc.*
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- SGS SA
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Merck KGaA
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Becton
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Dickinson and Company
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Wuxi Apptec
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- North American Science Associates Inc.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Nelson Laboratories, LLC
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Biomerieux SA
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Thermo Fisher Scientific
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Pacific Biolabs
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
11. References and Research Methodology
- References
- Research Methodology
- About us and Sales Contact




