PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672708

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672708
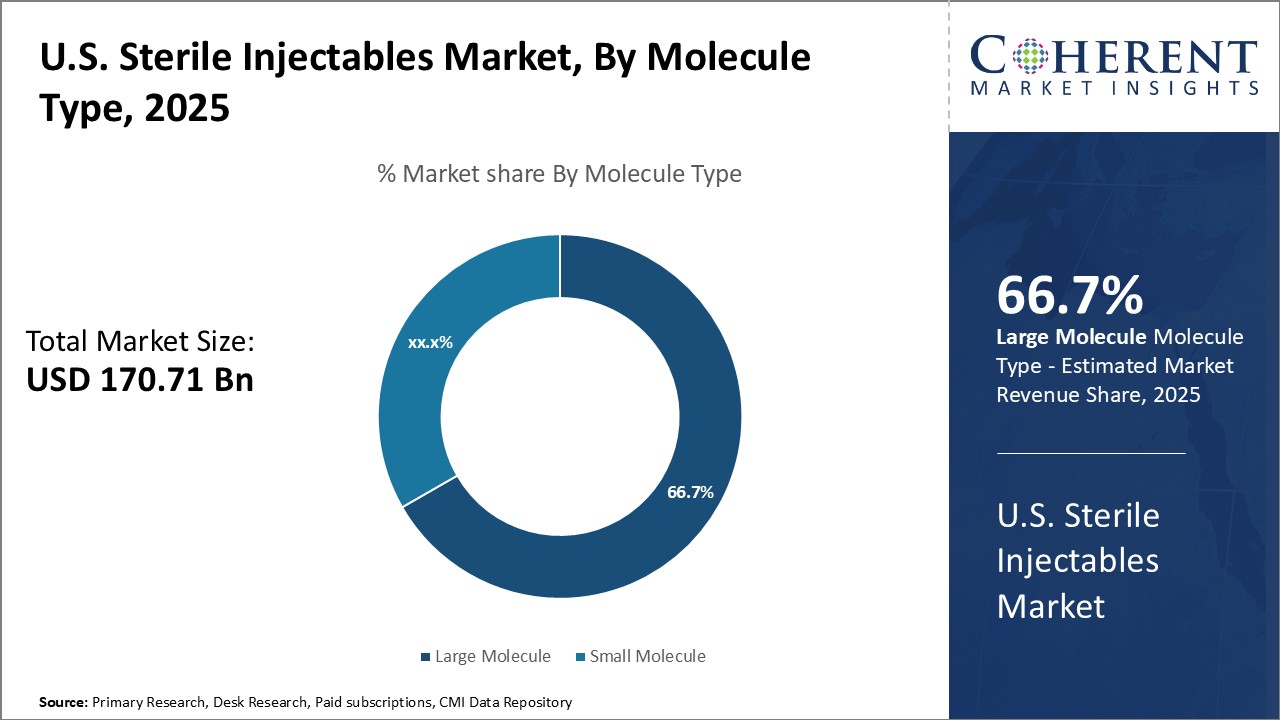
U.S. Sterile Injectables Market, By Molecule Type, By Drug Type, By Therapeutic Application, By Route of Administration, By Distribution Channel
Global U.S. Sterile Injectables Market is estimated to be valued at USD 170.71 Bn in 2025 and is expected to reach USD 328.67 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 170.71 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.80% | 2032 Value Projection: | USD 328.67 Bn |
Sterile injectables refer to parenteral drugs that are administered via various injection routes such as intravenous, intramuscular, subcutaneous, and others in their final sterile form for healing or preventing medical conditions. The rising prevalence of chronic diseases like cancer, diabetes, autoimmune disorders, and others and the increasing complexity of therapies have augmented the demand for sterile injectable drugs in the country. Moreover, the growing geriatric population who are more susceptible to such conditions also contributes to market expansion. Several pharmaceutical companies have been actively developing generic sterile injectable drugs to cater to this growing therapeutic segment in a cost-effective manner.
Market Dynamics:
The U.S. sterile injectables market is driven by factors such as the rising burden of chronic diseases, increasing adoption of biologics and biosimilars, growing healthcare spending, and expansion of drug manufacturing capabilities. However, the market growth can be hindered by stringent regulatory frameworks and the complex nature of sterile product development. Pharmaceutical firms now have significant opportunities to reduce production costs, improve process efficiencies, and ensure drug quality through advanced manufacturing technologies like continuous manufacturing and real-time release. Outsourcing sterile injectable production to well-established contract development and manufacturing organizations is also gaining traction.
Key Features of the Study:
This report provides in-depth analysis of the U.S. sterile injectables market, and provides market size (USD Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
Key companies covered as a part of this study include Baxter, AstraZeneca, Merck & Co., Inc., Novartis AG, Gilead Sciences, Inc., JHP Pharmaceuticals, Pfizer Inc., Fresenius Kabi Ag, CordenPharma, Recipharm AB, Hikma Pharmaceuticals PLC, and Eli Lilly and Company
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The U.S. sterile injectables market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Market Segmentation:
- By Molecule Type Insights (Revenue, USD Bn, 2020 - 2032)
- Large Molecule
- Small Molecule
- By Drug Type Insights (Revenue, USD Bn, 2020 - 2032)
- Monoclonal Antibody (mAbs)
- Cytokines
- Insulin
- Peptide Hormones
- Vaccines
- Others
- By Therapeutic Application Insights (Revenue, USD Bn, 2020 - 2032)
- Cancer
- Metabolic Disorder
- Cardiovascular Diseases
- Infectious Diseases
- Musculoskeletal Disorders
- Others
- By Route of Administration Insights (Revenue, USD Bn, 2020 - 2032)
- Subcutaneous
- Intravenous
- Intramuscular
- Others
- By Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Key Players Insights
- Baxter
- AstraZeneca
- Merck & Co., Inc.
- Novartis AG
- Gilead Sciences, Inc.
- JHP Pharmaceuticals
- Pfizer Inc.
- Fresenius Kabi Ag
- CordenPharma
- Recipharm AB
- Hikma Pharmaceuticals PLC
- Eli Lilly and Company
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- U.S. Sterile Injectables Market, By Molecule Type
- U.S. Sterile Injectables Market, By Drug Type
- U.S. Sterile Injectables Market, By Therapeutic Application
- U.S. Sterile Injectables Market, By Route of Administration
- U.S. Sterile Injectables Market, By Distribution Channel
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Epidemiology
4. U.S. Sterile Injectables Market, By Molecule Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Large Molecule
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Small Molecule
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
5. U.S. Sterile Injectables Market, By Drug Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Monoclonal Antibody (mAbs)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Cytokines
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Insulin
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Peptide Hormones
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Vaccines
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
6. U.S. Sterile Injectables Market, By Therapeutic Application, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Cancer
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Metabolic Disorder
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Cardiovascular Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Infectious Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Musculoskeletal Disorders
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
7. U.S. Sterile Injectables Market, By Route of Administration, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Subcutaneous
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Intravenous
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Intramuscular
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
8. U.S. Sterile Injectables Market, By Distribution Channel, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
9. Competitive Landscape
- Baxter
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- AstraZeneca
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Merck & Co., Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Novartis AG
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Gilead Sciences, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- JHP Pharmaceuticals
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Pfizer Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Fresenius Kabi Ag
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- CordenPharma
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Recipharm AB
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Hikma Pharmaceuticals PLC
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Eli Lilly and Company
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
10. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
11. References and Research Methodology
- References
- Research Methodology
- About us




