PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672840

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672840
Electronic Trial Master File (eTMF) Market, By Deployment Mode, By Functionality, By End User, By Geography
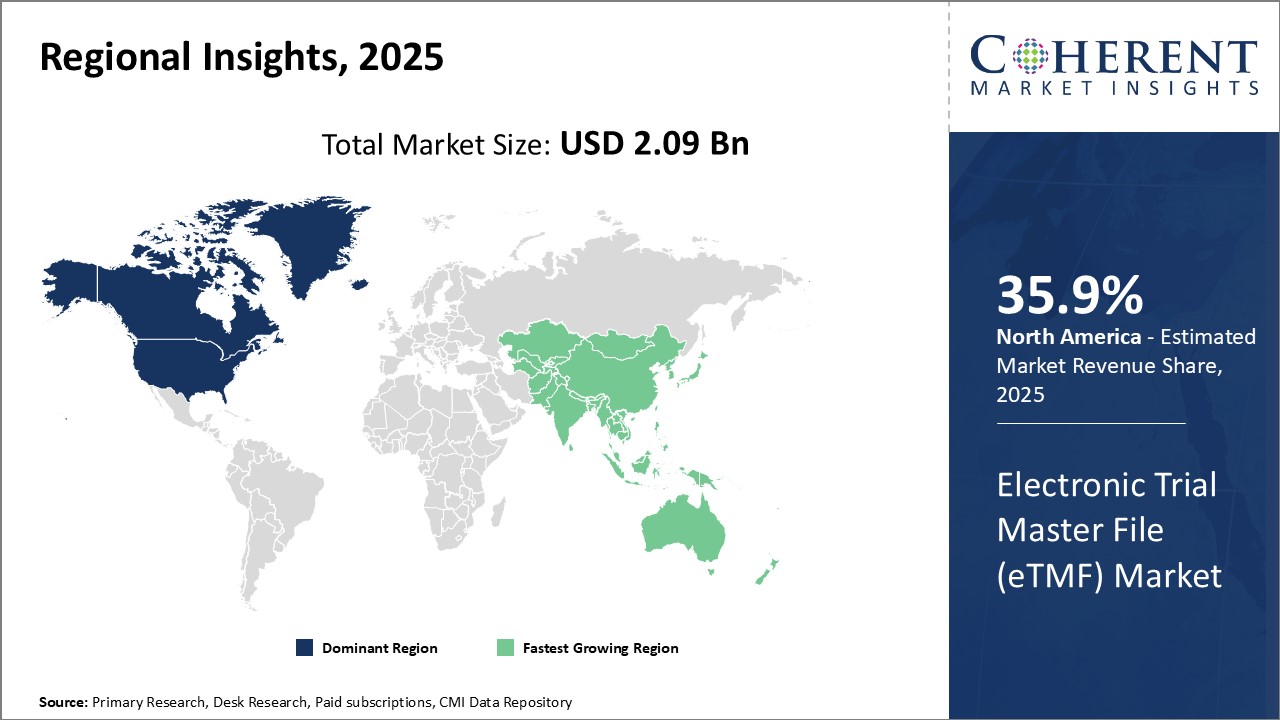
Global Electronic Trial Master File (eTMF) Market is estimated to be valued at USD 2.09 Bn in 2025 and is expected to reach USD 4.81 Bn by 2032, growing at a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.60% | 2032 Value Projection: | USD 4.81 Bn |
Global electronic trial master file (eTMF) market growth is driven by growing clinical trial processes across industries. eTMF refers to an integrated system that facilitates management of documents related to clinical trials in a digital/electronic format designed for ease of retrieval and archiving of documents. It helps maintain crucial trial-related documents in an organized manner and improve visibility throughout the lifecycle of a clinical trial. With increasing complexities in clinical trial processes and stricter regulations, there will be huge demand for eTMF solutions among organizations conducting trials to ensure compliance and improved oversight. The market growth is driven by factors such as regulatory compliance requirements, cost-efficiency of eTMF, and increasing R&D investments in drug development.
Market Dynamics:
Stringent regulatory guidelines around clinical trial documentation and data management across regions can drive the global electronic trial master file (eTMF) market growth. Regulatory bodies emphasize on maintaining proper documentation with electronic archiving to ensure data integrity. Rising R&D expenditure of pharmaceutical and biotechnology companies on new drug development can also drive the market growth. However, high implementation costs of eTMF systems especially for small and mid-sized companies can hamper the market growth. Cost benefits of eTMF along with better document security and accessibility can offer new growth opportunities. Vendors are also focusing on customizable technologies and cloud-based solutions to further enhance accessibility and lower operational costs.
Key features of the study:
This report provides in-depth analysis of the global electronic trial master file (eTMF) market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global electronic trial master file (eTMF) market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Veeva Systems Inc., Medidata Solutions, Inc., Oracle Corporation, Parexel International Corporation, IBM Watson Health, DrugDev (now part of Veeva), MasterControl, Inc., ArisGlobal LLC, Dassault Systemes, Trial Interactive, Signant Health, Forte Research Systems, Inc., Axiom Real-Time Metrics, eClinical Solutions, LLC, and Bioclinica, Inc.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global electronic trial master file (eTMF) market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global electronic trial master file (eTMF) market
Detailed Segmentation-
- By Deployment Mode Insights (Revenue, US$ Bn, 2020 - 2032)
- Cloud-based
- On-premises
- By Functionality Insights (Revenue, US$ Bn, 2020 - 2032)
- Document Management
- Workflow Management
- Reporting and Analytics
- Others
- By End User Insights (Revenue, US$ Bn, 2020 - 2032)
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic Research Institutions
- Regional Insights (Revenue, US$ Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Veeva Systems Inc.
- Medidata Solutions, Inc.
- Oracle Corporation
- Parexel International Corporation
- IBM Watson Health
- DrugDev (now part of Veeva)
- MasterControl, Inc.
- ArisGlobal LLC
- Dassault Systemes
- Trial Interactive
- Signant Health
- Forte Research Systems, Inc.
- Axiom Real-Time Metrics
- eClinical Solutions, LLC
- Bioclinica, Inc.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Deployment Mode
- Market Snippet, By Functionality
- Market Snippet, By End User
- Market Snippet, By Region
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Market Opportunities
- Regulatory Scenario
- Industry Trend
- Merger and Acquisitions
- New System Launches/Approvals
- Impact of COVID-19 Pandemic
4. Global Electronic Trial Master File (eTMF) Market, By Deployment Mode, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Cloud-based
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- On-premises
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
5. Global Electronic Trial Master File (eTMF) Market, By Functionality, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Document Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Workflow Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Reporting and Analytics
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Others
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
6. Global Electronic Trial Master File (eTMF) Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Pharmaceutical Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Biotechnology Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Contract Research Organizations (CROs)
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Academic Research Institutions
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
7. Global Electronic Trial Master File (eTMF) Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- North America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- U.S.
- Canada
- Europe
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- China
- India
- Japan
- ASEAN
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Israel
- GCC Countries
- Rest of the Middle East
- Africa
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country/Region, 2020-2032, (US$ Bn)
- South Africa
- North Africa
- Central Africa
8. Competitive Landscape
- Company Profiles
- Veeva Systems Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Medidata Solutions, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Oracle Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Parexel International Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- IBM Watson Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- DrugDev (now part of Veeva)
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- MasterControl, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- ArisGlobal LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Dassault Systemes
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Trial Interactive
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Signant Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Forte Research Systems, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Axiom Real-Time Metrics
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- eClinical Solutions, LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Bioclinica, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Veeva Systems Inc.
9. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
10. Research Methodology
- References
- Research Methodology
- About us and Sales Contact




