PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672877

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672877
Medical Device Vigilance Market, By Delivery Mode, By Application, By End User, By Geography
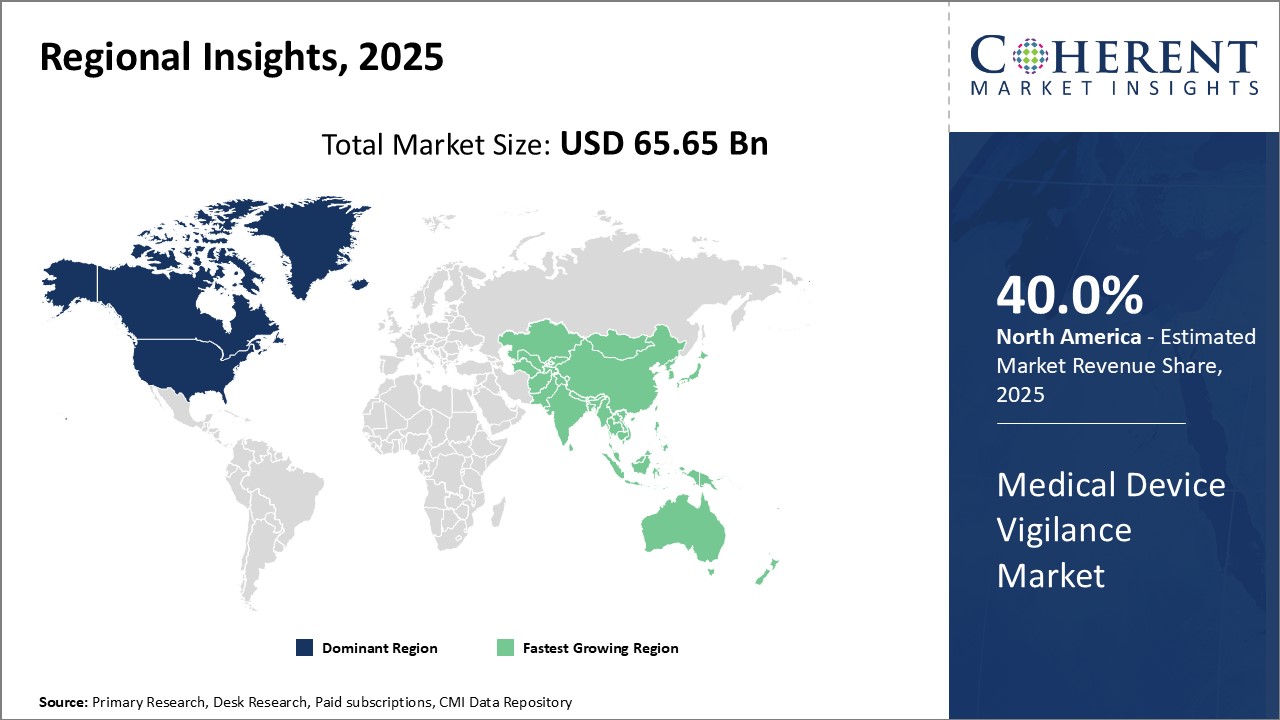
Global Medical Device Vigilance Market is estimated to be valued at USD 65.65 Bn in 2025 and is expected to reach USD 133.83 Bn by 2032, growing at a compound annual growth rate (CAGR) of 10.7% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 65.65 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.70% | 2032 Value Projection: | USD 133.83 Bn |
Rising incidence of chronic diseases and increasing emphasis on patient safety can boost demand for medical device vigilance across the world. Various regulatory bodies have implemented stringent norms and guidelines to ensure vigilance over medical devices post approval and marketing. With increasing complexity of medical devices, it is imperative for manufacturers to have robust vigilance systems for risk assessment, malfunction reporting and incident analysis. However, lack of harmonization and variation in regulatory framework across different regions can hamper the market growth. Ongoing research and development of innovative vigilance solutions can offer growth opportunities for market players in the near future.
Market Dynamics:
Growing burden of chronic diseases such as cancer, diabetes, and cardiovascular disorders globally has boosted demand for various types of medical devices. At the same time, numerous incidents related to device malfunctions, errors, and failures have emphasized the need for an effective post-market surveillance system. Stringent regulatory guidelines mandating implementation of vigilance procedures by manufacturers can drive the market growth. However, high costs associated with setting up and maintaining vigilance processes throughout the device lifecycle can hamper the market growth. Ongoing innovation in digital health technologies can offer new opportunities with integration of artificial intelligence and cloud computing in medical device vigilance solutions.
Key features of the study:
This report provides in-depth analysis of the global medical device vigilance market, and provides market size (USD Billion) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global medical device vigilance market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Philips, GE Healthcare, Siemens Healthineers, and Medtronic
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global medical device vigilance market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global medical device vigilance market
Detailed Segmentation-
- By Delivery Mode
- Cloud Based
- On-Premise
- By Application
- Therapeutics
- Diagnostics
- Surgical
- Research
- By End User
- Hospitals
- Diagnostic Imaging Center
- Contract Research Organization (CRO)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Others
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles:
- ZEINCRO
- AssurX, Inc.
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- Omnify Software, Inc.
- Medidata Solutions
- Vigilanz Corporation
- Qualio
- MasterControl
- Greenlight Guru
- eQMS
- BioClinica
- Medpace
- PAREXEL International
- Celerion
- Charles River Laboratories
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snapshot, By Delivery Mode
- Market Snapshot, By Application
- Market Snapshot, By End User
- Market Snapshot, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Impact Analysis
- Market Trends
- Key Developments
- Regulatory Scenario
- Acquisitions and Partnerships Scenario
- Funding and Investments
- PEST Analysis
- Porter's Analysis
4. Global Medical Device Vigilance Market- Impact of Coronavirus (COVID-19) Pandemic
- Overall Impact
- Government Initiatives
- COVID-19 Impact on the Market
5. Global Medical Device Vigilance Market, By Delivery Mode, 2020 - 2032, (USD Bn)
- Overview
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Cloud Based
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- On-Premise
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
6. Global Medical Device Vigilance Market, By Application, 2020 - 2032, (USD Bn)
- Overview
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Therapeutics
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Diagnostics
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Surgical
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Research
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
7. Global Medical Device Vigilance Market, By End User, 2020 - 2032, (USD Bn)
- Overview
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospitals
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Diagnostic Imaging Center
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Contract Research Organization (CRO)
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Business Process Outsourcing (BPO)
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Original Equipment Manufacturers (OEM)
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Overview
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
8. Global Medical Device Vigilance Market, By Region, 2020 - 2032, (USD Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, For Region, 2021-2032
- Regional Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, (USD Bn)
- U.S.
- Canada
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, (USD Bn)
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, (USD Bn)
- U.K.
- Germany
- Italy
- France
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, (USD Bn)
- China
- India
- Japan
- ASEAN
- Australia
- South Korea
- Rest of Asia Pacific
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, (USD Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Delivery Mode, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020 - 2032, (USD Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020 - 2032, (USD Bn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Company Profiles
- ZEINCRO
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- AssurX, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- Sparta Systems
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- Oracle Corporation
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- Xybion Corporation
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- Sarjen Systems Pvt. Ltd.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Market Strategies
- Omnify Software, Inc.
- Medidata Solutions
- Vigilanz Corporation
- Qualio
- MasterControl
- Greenlight Guru
- eQMS
- BioClinica
- Medpace
- PAREXEL International
- Celerion
- Charles River Laboratories
- ZEINCRO
10. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
11. References and Research Methodology
- References
- Research Methodology
- About Us and Sales Contact




