PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672819

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672819
Influenza Vaccines Market, By Vaccine Type, By Valency, By Route of Administration, By Age Group, By Technology, By Distribution Channel, By Geography
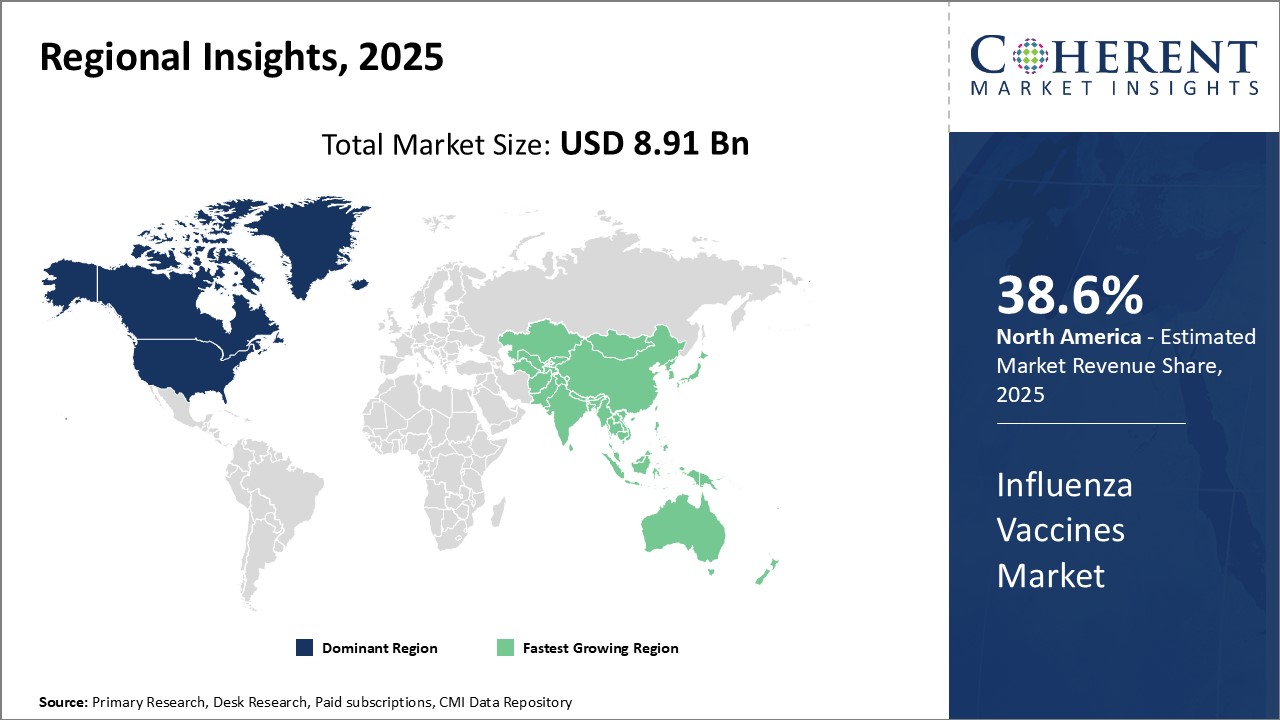
Global Influenza Vaccines Market is estimated to be valued at USD 8.91 Bn in 2025 and is expected to reach USD 14.59 Bn by 2032, growing at a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.91 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.30% | 2032 Value Projection: | USD 14.59 Bn |
Influenza vaccines have been an important element of preventive healthcare for decades. The influenza viruses have the ability to mutate and evolve regularly, necessitating the development of updated vaccines each year. The vaccines work to boost the body's immune response to these changing viruses. They are designed to immunize against the three or four strains (depending on the vaccine type used for a particular flu season) that surveillance and other scientific studies have determined are most likely to be in circulation for the upcoming flu season in any given region. The global influenza vaccines market has witnessed steady growth over the past few years due to the growing awareness about seasonal vaccination, introduction of novel technologies, expansion of vaccination guidelines by healthcare organizations, and rising government support worldwide.
Market Dynamics:
The global influenza vaccines market growth is driven by various factors such as the increasing prevalence of influenza, growing adoption of vaccination guidelines set by international health organizations, rising awareness about vaccination, new product launches, growing preference for recombinant technology vaccines, and expansion in geographical reach of major players. However, the market growth can be challenging due to high R&D costs involved for development of quadrivalent influenza vaccines, stringent regulatory guidelines for approval of new vaccines and vaccine resistance arising due to antigenic drift of influenza virus strains. The market players see opportunities in emerging countries owing to improving healthcare infrastructure and rising disposable incomes. Manufacturers are also working on the development of universal influenza vaccine with durable protection against multiple flu strains.
Key Features of the Study:
- This report provides an in-depth analysis of the global influenza vaccines market, and provides market size (USD BN) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year.
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market.
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players.
- It profiles key players in the global influenza vaccines market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies.
- Key companies covered as a part of this study includes Seqirus, GlaxoSmithKline plc, Sanofi, AstraZeneca, FluGen Inc., Moderna Inc., Biocryst Pharmaceuticals Inc., CPL Biologicals Pvt. Ltd., CureVac AG, OSIVAX, Solaris Vaccines, Pfizer, CSL, Daiichi Sankyo, Bharat Biotech, and Sinovac Biotech.
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics.
- The global influenza vaccines market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts.
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global influenza vaccines market.
Market Segmentation
- Vaccine Type:
- Inactivated
- Live Attenuated
- Valency:
- Quadrivalent
- Trivalent
- Route of Administration:
- Injection
- Nasal Spray
- Age Group:
- Pediatrics
- Adults
- Technology:
- Egg-Based Flu Vaccines
- Cell Culture-Based Flu Vaccines
- Recombinant Flu Vaccines
- Distribution Channel:
- Public
- Private
- Regional:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles:
- Seqirus
- GlaxoSmithKline plc
- Sanofi
- AstraZeneca
- FluGen Inc.
- Moderna Inc.
- Biocryst Pharmaceuticals Inc.
- CPL Biologicals Pvt. Ltd.
- CureVac AG
- OSIVAX
- Solaris Vaccines
- Pfizer
- CSL
- Daiichi Sankyo
- Bharat Biotech
- Sinovac Biotech
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snapshot, By Vaccine Type
- Market Snapshot, By Valency
- Market Snapshot, By Route of Administration
- Market Snapshot, By Age Group
- Market Snapshot, By Technology
- Market Snapshot, By Distribution Channel
- Market Snapshot, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Impact Analysis
- Key Developments
- Industry Trends
- Regulatory Scenario
- Recent Product Approval/Launches
- PEST Analysis
- Porter's Analysis
4. Global Influenza Vaccines Market- Impact of Coronavirus (COVID-19) Pandemic
- Impact on Demand
- Impact on Healthcare
- Epidemiology
5. Global Influenza Vaccines Market, By Vaccine Type, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Inactivated
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Live Attenuated
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD BN)
6. Global Influenza Vaccines Market, By Valency, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Quadrivalent
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Trivalent
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD BN)
7. Global Influenza Vaccines Market, By Route of Administration, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Injection
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Nasal Spray
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD BN)
8. Global Influenza Vaccines Market, By Age Group, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Pediatrics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Adults
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
9. Global Influenza Vaccines Market, By Technology, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Egg-Based Flu Vaccines
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Cell Culture-Based Flu Vaccines
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Recombinant Flu Vaccines
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
10. Global Influenza Vaccines Market, By Distribution Channel, 2020 - 2032, (USD BN)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2020 - 2032
- Segment Trends
- Public
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
- Private
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, (USD BN)
11. Global Influenza Vaccines Market, By Region, 2020 - 2032 (USD BN)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032(%)
- Y-o-Y Growth Analysis, For Regions, 2020 - 2032
- Regional Trends
- North America
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Country, 2020 - 2032 (USD BN)
- U.S.
- Canada
- Latin America
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Country, 2020 - 2032 (USD BN)
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Europe
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Country, 2020 - 2032 (USD BN)
- Germany
- U.K.
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Country, 2020 - 2032 (USD BN)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Country, 2020 - 2032 (USD BN)
- GCC
- Israel
- Rest of Middle East
- Africa
- Market Size and Forecast, By Vaccine Type, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Valency, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Route of Administration, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Age Group, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Technology, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Distribution Channel, 2020 - 2032 (USD BN)
- Market Size and Forecast, By Region/Country, 2020 - 2032 (USD BN)
- South Africa
- Central Africa
- North Africa
12. Competitive Landscape
- Heat Map Analysis
- Market Share Analysis
- CSL Seqirus
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- GlaxoSmithKline plc
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Sanofi
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- AstraZeneca
- FluGen, Inc.
- Moderna, Inc.
- Biocryst Pharmaceuticals Inc.
- CPL Biologicals Pvt. Ltd.
- CureVac AG
- OSIVAX
- Solaris Vaccines
- Pfizer
- Daiichi Sankyo
- Bharat Biotech
- Sinovac Biotech
- Solaris Vaccines
- CSL Seqirus
13. Analyst View
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
14. References and Research Methodology
- References
- Research Methodology
- About us and Sales Contact




