PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1708377

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1708377
Global External Ventricular Drain Market, By Application, By Patient Type, By End User, By Geography .
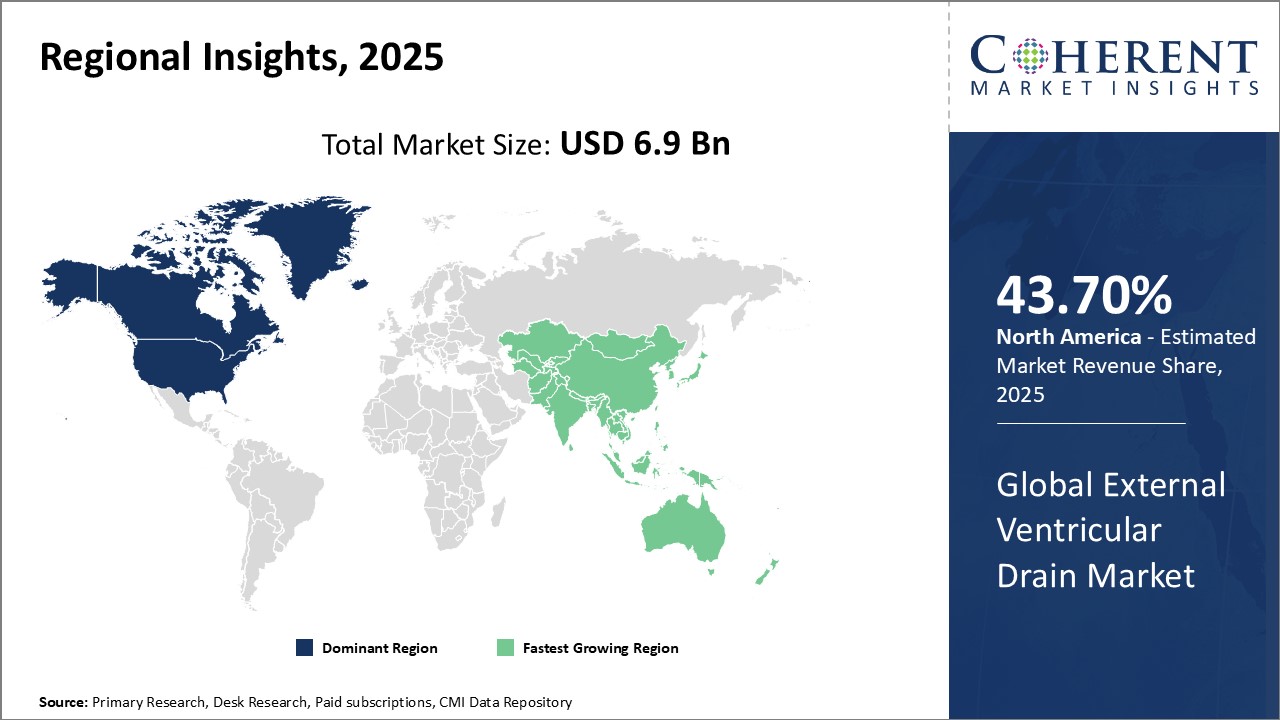
Global External Ventricular Drain Market is estimated to be valued at USD 6.9 Bn in 2025 and is expected to reach USD 10.24 Bn by 2032, growing at a compound annual growth rate (CAGR) of 5.8% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 6.9 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.80% | 2032 Value Projection: | USD 10.24 Bn |
An external ventricular drain (EVD) is a soft catheter that is inserted via a burr hole into the anterior horn of the lateral ventricle and connected to a closed sterile system to allow for temporary drainage of cerebrospinal fluid (CSF) and/or monitoring of intracranial pressure. Indications for external ventricular drainage are: to relieve raised intracranial pressure, to divert infected CSF, to divert bloodstained CSF following neurosurgery/haemorrhage, to divert the flow of CSF, and to monitor intracranial pressure. External drainage and monitoring is the standard of care for temporarily controlling intracranial pressure by draining cerebrospinal fluid externally from the body. External drainage and monitoring is the temporary drainage of cerebrospinal fluid (CSF) from the lateral ventricles of the brain, or the lumbar space of the spine, into an external collection bag. An external ventricular drainage (EVD) system drains CSF by using a combination of gravity and intercerebral pressure. The drainage rate depends on the height at which the EVD system is placed relative to the patient's anatomy.
Market Dynamics:
Increasing research and development (R&D) activities is expected to drive the growth of the global external ventricular drain market over the forecast period. For instance, in June 2024, Karolinska University Hospital, a hospital based in Sweden, initiated a clinical trial titled "Novel Diagnostic Methods to Identify External Ventricular Drain Associated Infections". This study will evaluate three novel diagnostic methods for rapid direct bacterial detection in CSF, in order to assess, if these can be used to guide antibiotic treatment in neurocritically ill patients, as compared to CSF bacterial cultures. The study is expected to get completed by December 31, 2025.
Key Features of the Study:
- This report provides an in-depth analysis of the global external ventricular drain market, and provides market size (US$ Bn) and compound annual growth rate (CAGR %) for the forecast period (2025-2032), considering 2024 as the base year
- It elucidates potential revenue growth opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global external ventricular drain market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
- Key companies covered as a part of this study include Spiegelberg GmbH & Co. KG, Medtronic, Sophysa, Neuromedex GmbH, Integra LifeSciences, Moller Medical GmbH, B. Braun SE and Fuji Systems
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- Global external ventricular drain market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global external ventricular drain market
Detailed Segmentation:
- Global External Ventricular Drain Market, By Application
- Traumatic Brain Injury
- Subarachnoid Hemorrhage
- Intracerebral Hemorrhage
- Other (Non-traumatic Hydrocephalus Condition)
- Global External Ventricular Drain Market, By Patient Type
- Pediatric
- Adult
- Global External Ventricular Drain Market, By End User
- Hospitals
- Clinics
- Ambulatory Surgery Centers
- Others
- Global External Ventricular Drain Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Company Profiles
- Spiegelberg GmbH & Co. KG
- Medtronic
- Sophysa
- Neuromedex GmbH
- Integra LifeSciences
- Moller Medical GmbH
- Braun SE
- Fuji Systems
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Application
- Market Snippet, By Patient Type
- Market Snippet, By End User
- Market Snippet, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Increasing prevalence of traumatic injuries
- Risk of post-surgery infections
- Emerging markets in the Asia Pacific region
- Key Highlights
- Recent Trends
- PEST Analysis
- Recent Product Lauches/Approvals
- Mergers, Acquisitions, and Collaborations
4. Global External Ventricular Drain Market - Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global External Ventricular Drain Market, By Application, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Traumatic Brain Injury
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Subarachnoid Hemorrhage
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Intracerebral Hemorrhage
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Other ((Non-traumatic Hydrocephalus Condition)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
6. Global External Ventricular Drain Market, By Patient Type, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Pediatric
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Adult
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
7. Global External Ventricular Drain Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Ambulatory Surgical Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Hospice Care Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
8. Global External Ventricular Drain Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, For Region, 2021 - 2032
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Patient Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020-2032,(US$ Bn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Company Profile
- Spiegelberg GmbH & Co. KG
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Medtronic
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Sophysa
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Neuromedex GmbH
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Integra LifeSciences
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Moller Medical GmbH
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Braun SE
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Fuji Systems
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
10. Section
- References
- Research Methodology
- About us




