PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705779

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1705779
Protein Stability Analysis Market, By Product, By Technique, By End User, By Geography
Global Protein Stability Analysis Market is estimated to be valued at USD 2.44 Bn in 2025 and is expected to reach USD 5.23 Bn by 2032, growing at a compound annual growth rate (CAGR) of 11.55% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.44 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.55% | 2032 Value Projection: | USD 5.23 Bn |
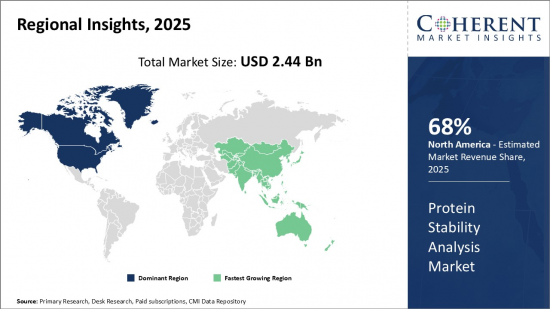
Protein stability analysis involves studying the physical and chemical stability of protein therapeutics during development and storage. Proteins can undergo various degradations such as aggregation, fragmentation, deamidation, and oxidation when exposed to stress conditions like heat, light, and freeze-thaw cycles. This affects the safety, efficacy, and shelf life of protein drugs. Protein stability analysis helps evaluate the conformational changes in proteins under various conditions and identify the degradants to optimize formulation and storage parameters. The results aid in developing protein biologics with enhanced stability profiles. Various analytical techniques like differential scanning calorimetry, light scattering, and chromatography are utilized for stability indication and characterization.. With growing biologics revenues, protein stability analysis market is expected to expand significantly in the near future. The market is also influenced by the increasing number of recombinant protein therapeutics and biologics, as well as the need for more accurate and reliable methods to assess protein stability.
Market Dynamics:
The protein stability analysis market is driven by the rising demand for biologics and their growing contribution to the pharmaceutical pipeline. Biologics development requires extensive stability testing during various stages from pre-clinical to commercialization. Regulatory mandates from authorities like Food and Drug Administration (FDA) for stability testing and assignment of expiration dates fuels the market growth. However, high costs that are associated with analytical equipment and assay development poses a challenges. Meanwhile, outsourcing of stability studies to contract research organizations presents opportunities for market players. Also, automation and advanced techniques for high-throughput stability screening will further support the market expansion going forward.
Key features of the study:
- This report provides in-depth analysis of the protein stability analysis market, and provides market size (US$ Billion) and compound annual growth rate (CAGR %) for the forecast period (2025-2032), considering 2024 as the base year.
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market.
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players.
- It profiles key players in the protein stability analysis market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies.
- Key companies covered as a part of this study include Thermo Fisher Scientific, Inc, Enzo Biochem, Inc., PerkinElmer Inc., NanoTemper, GE Healthcare, HORIBA, Ltd., Malvern Panalytical Ltd., Agilent Technologies, Inc., SETARAM Instrumentation, Unchained Labs, Waters Corporation, and Applied Photophysics Ltd.
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics.
- The protein stability analysis market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts.
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the protein stability analysis market.
Market Segmentation:
- Protein Stability Analysis Market, By Product
- Reagents and Assay Kits
- Instruments
- Consumables and Accessories
- Software
- Service
- Protein Stability Analysis Market, By Technique
- Chromatography
- Spectroscopy
- Differential Scanning Calorimetry (DSC)
- Differential Scanning Fluorimetry (DSF)
- Dynamic Light Scattering (DLS)
- Others
- Protein Stability Analysis Market, By End User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic Research Institutes
- Protein Stability Analysis Market, By Region
- North America
- Latin America
- Europe
- Asia Pacific
- Middle East and Africa
- Company Profiles
- Thermo Fisher Scientific
- Enzo Biochem, Inc.
- PerkinElmer Inc.
- NanoTemper
- GE Healthcare
- HORIBA Ltd.
- Malvern Panalytical Ltd.
- Agilent Technologies, Inc.
- SETARAM Instrumentation
- Unchained Labs
- Waters Corporation
- Applied Photophysics Ltd.
Table of Content:
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Overview
- Report Description
- Market Definition and Scope
- Executive Summary
- Protein Stability Analysis Market, By Product
- Protein Stability Analysis Market, By Technique
- Protein Stability Analysis Market, By End-use
- Protein Stability Analysis Market, By Region
- Coherent Opportunity Map (COM)
3. Key Industry Insights
- Market Dynamics
- Drivers
- Growing demand from pharmaceutical industry
- Adoption in biopharmaceutical manufacturing
- Restraints
- Oppertunities
- Impact Analysis
- Pest Analysis
- Porters Analysis
- Merger, Acquisition and Collaboration Scenario
- Recent Product Approvals/Launches
- Company Market Share Analysis
4. Protein Stability Analysis Market (Impact of Coronavirus COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Impact Protein Stability Analysis Market
5. Protein Stability Analysis Market, By Product, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Reagents And Assay Kits, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Instruments, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth,, 2020 - 2032, ( US$ Billion)
- Consumables And Accessories, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Software, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Service, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
6. Protein Stability Analysis Market, By Technique, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Chromatography, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Spectroscopy, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Differential Scanning Calorimetry (dsc), 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Differential Scanning Fluorimetry (dsf), 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Dynamic Light Scattering (dls), 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth 2020 - 2032, ( US$ Billion)
- Others, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
7. Protein Stability Analysis Market, By End-use, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Size and Forecast, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Pharmaceutical & Biotechnology Companies, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Contract Research Organizations, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- Academic Research Institutes, 2020 - 2032( US$ Billion)
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
8. Protein Stability Analysis Market, By Region, 2020 - 2032 ( US$ Billion)
- Introduction
- Market Share Analysis, 2020 - 2032, ( US$ Billion)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- By Region
- Introduction
- Market Size and Forecast and Y-o-Y Growth, 2020 - 2032, ( US$ Billion)
- North America
- Introduction
- Market Size and Forecast, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By Technique, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By End-use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, By Country, 2020 - 2032, ( US$ Billion)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Technique, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth ,By End-use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth, By Country, 2020 - 2032, ( US$ Billion)
- U.K.
- Germany
- Italy
- France
- Spain
- Russia
- Rest Of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast and Y-o-Y Growth, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth By Technique, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast , and Y-o-Y Growth By End-use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast , and Y-o-Y Growth By Country, 2020 - 2032, ( US$ Billion)
- China
- India
- Japan
- Australia
- South Korea
- Asean
- Rest Of Asia Pacific
- Middle East & Africa
- Introduction
- Market Size and Forecas and Y-o-Y Growth t, By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast and Y-o-Y Growth By Technique, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast , and Y-o-Y Growth By End-use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, and Y-o-Y Growth By Country, 2020 - 2032, ( US$ Billion)
- Gcc
- Israel
- Rest Of Middle East
- Latin America
- Introduction
- Market Size and Forecast, , and Y-o-Y Growth By Product, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, and Y-o-Y Growth, By Technique, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, and Y-o-Y Growth, By End-use, 2020 - 2032, ( US$ Billion)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020 - 2032, ( US$ Billion)
- Brazil
- Mexico
- Argentina
9. Company Profiles - Protein Stability Analysis Market
- Thermo Fisher Scientific, Inc
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Enzo Biochem, Inc.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- PerkinElmer Inc.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- NanoTemper
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- GE Healthcare
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- HORIBA, Ltd.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Malvern Panalytical Ltd.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Agilent Technologies, Inc.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- SETARAM Instrumentation
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Unchained Labs
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Waters Corporation
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
- Applied Photophysics Ltd.
- Company Overview
- Product/Service Portfolio
- Financial Performance
- Recent Developments/Updates
- Strategic Overview
10. References and Research Methodology
- References
- Research Methodology
- About us and Sales Contact




