PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1706034

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1706034
Rapid Medical Diagnostic Kits Market, By Test Type, By End User By Region (North America, Europe, Asia Pacific, Latin America, Middle East and Africa)
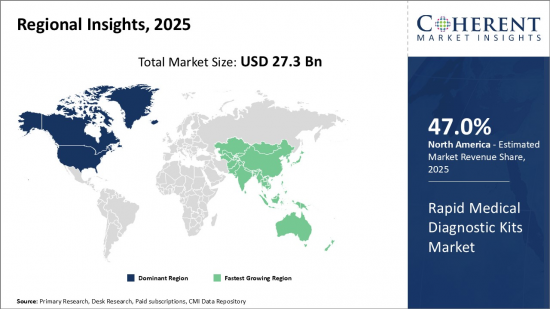
Global Rapid Medical Diagnostic Kits Market is estimated to be valued at USD 27.3 Bn in 2025 and is expected to reach USD 51.53 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.5% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 27.3 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.50% | 2032 Value Projection: | USD 51.53 Bn |
Rapid diagnostic tests (RDTs) refer to a group of diagnostics categorized by performance characteristics rather than the specific analyte or test platform. Such assays have relatively short performance times, provide results to inform clinical decision making, and enable management at the point-of-care (POC). RDTs are available in a variety of test formats and platforms for various detection targets. RDTs are designed for detecting pathogen-specific antigens or nucleic acid sequences, as well as host antibody responses against certain pathogens.
Market Dynamics:
Increasing research and development activities by the market players to develop novel product for the rapid diagnosis of infectious diseases. Moreover, the increasing prevalence of infectious diseases globally is expected to drive market growth over the forecast period. For instance, according to the data published by the Center for Disease Control and Prevention in 2020, the number of new tuberculosis cases was 8,916, and the number of new Lyme disease cases was 34,945.
Rapid Medical Diagnostic Kits Market Segmentation:
- By Test Type
- Blood glucose monitoring test kits
- Pregnancy test kits
- Infectious disease test kits
- Coagulation monitoring test kits
- Others
- By Sample Type
- Blood tests
- Urine tests
- Saliva tests
- Others
- By End User
- Hospitals and clinics
- Home care settings
- Diagnostic laboratories
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles
- Abbott Laboratories
- Roche
- Becton, Dickinson and Company
- Thermo Fisher Scientific
- Quidel Corporation
- bioMerieux SA
- Meridian Bioscience, Inc.
- PerkinElmer, Inc.
- LumiraDx
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Rapid Medical Diagnostic Kits Market, By Test Type
- Rapid Medical Diagnostic Kits Market, By Sample Type
- Rapid Medical Diagnostic Kits Market, By End User
- Rapid Medical Diagnostic Kits Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Growing Prevalence of Infectious Diseases And Epidemiology
- Faster Results of Rapid Test Kits
- Restraints
- Issues Regarding Accuracy and Reliability
- Opportunities
- New Product Launches
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product launch/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
4. Rapid Medical Diagnostic Kits Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Rapid Medical Diagnostic Kits Market, By Test Type, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Blood glucose monitoring test kits
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Pregnancy test kits
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Infectious disease test kits
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Coagulation monitoring test kits
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
6. Rapid Medical Diagnostic Kits Market, By Sample Type, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Blood tests
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Urine tests
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Saliva tests
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Bn)
7. Rapid Medical Diagnostic Kits Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025and 2032 (%)
- Y-o-Y Growth Analysis, 2021- 2032
- Segment Trends
- Hospitals and clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Billion)
- Home care settings
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Billion)
- Diagnostic laboratories
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Billion)
8. Rapid Medical Diagnostic Kits Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Country, 2025and 2032 (%)
- Y-o-Y Growth Analysis, For Country 2021-2032
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- U.K.
- Germany
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- China
- Japan
- India
- Australia
- ASEAN
- South Korea
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Bn)
- GCC
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Test Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Sample Type, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032,(US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Region/Country, 2020-2032,(US$ Bn)
- South Africa
- North Africa
- Central Africa
9. Competitive Landscape
- Abbott Laboratories
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Roche
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Becton, Dickinson and Company
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Thermo Fisher Scientific
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Quidel Corporation
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- bioMerieux SA
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Meridian Bioscience, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- PerkinElmer, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- LumiraDx
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
10. Section
- Research Methodology
- About us




