PUBLISHER: Allied Market Research | PRODUCT CODE: 1641735

PUBLISHER: Allied Market Research | PRODUCT CODE: 1641735
Lateral Flow Conjugate Release Pad Market By Material Type , By Application By End User : Global Opportunity Analysis and Industry Forecast, 2024-2033
Lateral Flow Conjugate Release Pad Market
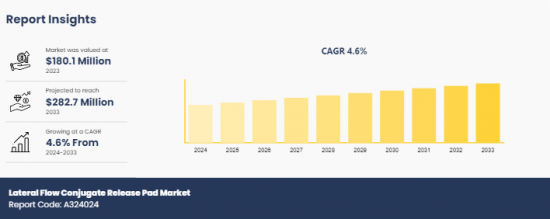
The lateral flow conjugate release pad market was valued at $180.1 million in 2023 and is projected to reach $282.7 million by 2033, growing at a CAGR of 4.6% from 2024 to 2033.
A lateral flow conjugate release pad is an object utilized in lateral flow immunoassay (LFA) to store and release reagents during the diagnostic process. LFA is an immunochromatography-based diagnostic test that performs qualitative detection of target substances instantly. A key characteristic of the release pad is its ability to allow the flow of reagent at the correct time during the diagnostic process. The object finds applications in medical diagnostics, food safety, veterinary analysis, and environmental testing.
Increase in the popularity of point-of-care testing has fueled the need for LFA devices in various healthcare settings, which acts as a key driver of the lateral flow conjugate release pad market. In addition, as consumers shift toward self-testing kits for various diagnoses, the market development is being augmented notably owing to the critical role of lateral flow conjugate release pads in self-testing kits. Currently, the incorporation of nanoparticles such as quantum dots or carbon nanotubes into the conjugates is trending. These nanoparticles enhance the sensitivity & specificity of release pads, which results in high signal intensity, quick reaction times, and low detection limits.
However, the usage of nanoparticles or advanced materials increases the costs of lateral flow conjugate release pads, which restricts their widespread adoption and hampers the market development. Moreover, rise in concerns pertaining to the disposal of release pads and the environmental impact associated with them is restraining the market growth. Contrarily, as scientists are exploring the development of LFA devices that detect multiple conditions through a single sample, the lateral flow conjugate release pad market is anticipated to witness remunerative opportunities in the future. According to Sartorius-a German pharmaceutical company-LFA multiplexing or screening of a single sample for multiple targets is poised to be a significant breakthrough in the field of medical diagnosis. This is attributed to its ability to save time, simplify the testing process, and reduce the requirement for samples & reagents.
Segment Review
The lateral flow conjugate release pad market is segmented into material type, application, end user, and region. On the basis of material type, the market is divided into glass fiber, cellulose fiber, polyester, and other materials. As per application, it is classified into clinical testing, food safety and environmental testing, veterinary diagnostics, and others. By end user, it is categorized into hospitals & clinics, diagnostic laboratories, pharmaceutical & biotechnology companies, and others. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Key Findings
On the basis of material type, the glass fiber segment is expected to dominate the market during the forecast period.
As per application, the clinical testing segment is anticipated to be the highest shareholder from 2024 to 2033.
By end user, the hospitals & clinics segment is predicted to acquire a notable stake in the market throughout the forecast period.
Region wise, North America is projected to be the highest revenue generator by 2033.
Competition Analysis
The major players in the global lateral flow conjugate release pad market include Danaher Corporation, AntiTeck, Merck KGaA, Axiflow Biotech Private Limited, Ahlstrom, Axiva, Porex, Cytodiagnostics Inc, Advanced Microdevices Pvt. Ltd., and Asahi Kasei. These major players have adopted various key development strategies such as business expansion, new product launches, and partnerships to strengthen their foothold in the competitive market.
Additional benefits you will get with this purchase are:
- Quarterly Update and* (only available with a corporate license, on listed price)
- 5 additional Company Profile of client Choice pre- or Post-purchase, as a free update.
- Free Upcoming Version on the Purchase of Five and Enterprise User License.
- 16 analyst hours of support* (post-purchase, if you find additional data requirements upon review of the report, you may receive support amounting to 16 analyst hours to solve questions, and post-sale queries)
- 15% Free Customization* (in case the scope or segment of the report does not match your requirements, 15% is equivalent to 3 working days of free work, applicable once)
- Free data Pack on the Five and Enterprise User License. (Excel version of the report)
- Free Updated report if the report is 6-12 months old or older.
- 24-hour priority response*
- Free Industry updates and white papers.
Possible Customization with this report (with additional cost and timeline, please talk to the sales executive to know more)
- Regulatory Guidelines
- Additional company profiles with specific to client's interest
- Additional country or region analysis- market size and forecast
- Expanded list for Company Profiles
- Historic market data
- Key player details (including location, contact details, supplier/vendor network etc. in excel format)
Key Market Segments
By Material Type
- Glass Fiber
- Cellulose Fiber
- Polyester
- Other Materials
By Application
- Clinical Testing
- Food Safety and Environmental Testing
- Veterinary Diagnostics
- Others
By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Pharmaceutical and Biotechnology Companies
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- LAMEA
- Brazil
- Saudi Arabia
- South Africa
- Rest of LAMEA
Key Market Players:
- Danaher Corporation
- AntiTeck
- Merck KGaA
- Axiflow Biotech Private Limited
- Ahlstrom
- Axiva
- Porex
- Cytodiagnostics Inc
- Advanced Microdevices Pvt. Ltd.
- Asahi Kasei
TABLE OF CONTENTS
CHAPTER 1: INTRODUCTION
- 1.1. Report Description
- 1.2. Key Market Segments
- 1.3. Key Benefits
- 1.4. Research Methodology
- 1.4.1. Primary Research
- 1.4.2. Secondary Research
- 1.4.3. Analyst Tools and Models
CHAPTER 2: EXECUTIVE SUMMARY
- 2.1. CXO Perspective
CHAPTER 3: MARKET LANDSCAPE
- 3.1. Market Definition and Scope
- 3.2. Key Findings
- 3.2.1. Top Investment Pockets
- 3.2.2. Top Winning Strategies
- 3.3. Porter's Five Forces Analysis
- 3.3.1. Bargaining Power of Suppliers
- 3.3.2. Threat of New Entrants
- 3.3.3. Threat of Substitutes
- 3.3.4. Competitive Rivalry
- 3.3.5. Bargaining Power among Buyers
- 3.4. Market Dynamics
- 3.4.1. Drivers
- 3.4.2. Restraints
- 3.4.3. Opportunities
CHAPTER 4: LATERAL FLOW CONJUGATE RELEASE PAD MARKET, BY MATERIAL TYPE
- 4.1. Market Overview
- 4.1.1 Market Size and Forecast, By Material Type
- 4.2. Glass Fiber
- 4.2.1. Key Market Trends, Growth Factors and Opportunities
- 4.2.2. Market Size and Forecast, By Region
- 4.2.3. Market Share Analysis, By Country
- 4.3. Cellulose Fiber
- 4.3.1. Key Market Trends, Growth Factors and Opportunities
- 4.3.2. Market Size and Forecast, By Region
- 4.3.3. Market Share Analysis, By Country
- 4.4. Polyester
- 4.4.1. Key Market Trends, Growth Factors and Opportunities
- 4.4.2. Market Size and Forecast, By Region
- 4.4.3. Market Share Analysis, By Country
- 4.5. Other Materials
- 4.5.1. Key Market Trends, Growth Factors and Opportunities
- 4.5.2. Market Size and Forecast, By Region
- 4.5.3. Market Share Analysis, By Country
CHAPTER 5: LATERAL FLOW CONJUGATE RELEASE PAD MARKET, BY APPLICATION
- 5.1. Market Overview
- 5.1.1 Market Size and Forecast, By Application
- 5.2. Clinical Testing
- 5.2.1. Key Market Trends, Growth Factors and Opportunities
- 5.2.2. Market Size and Forecast, By Region
- 5.2.3. Market Share Analysis, By Country
- 5.3. Food Safety And Environmental Testing
- 5.3.1. Key Market Trends, Growth Factors and Opportunities
- 5.3.2. Market Size and Forecast, By Region
- 5.3.3. Market Share Analysis, By Country
- 5.4. Veterinary Diagnostics
- 5.4.1. Key Market Trends, Growth Factors and Opportunities
- 5.4.2. Market Size and Forecast, By Region
- 5.4.3. Market Share Analysis, By Country
- 5.5. Others
- 5.5.1. Key Market Trends, Growth Factors and Opportunities
- 5.5.2. Market Size and Forecast, By Region
- 5.5.3. Market Share Analysis, By Country
CHAPTER 6: LATERAL FLOW CONJUGATE RELEASE PAD MARKET, BY END USER
- 6.1. Market Overview
- 6.1.1 Market Size and Forecast, By End User
- 6.2. Hospitals And Clinics
- 6.2.1. Key Market Trends, Growth Factors and Opportunities
- 6.2.2. Market Size and Forecast, By Region
- 6.2.3. Market Share Analysis, By Country
- 6.3. Diagnostic Laboratories
- 6.3.1. Key Market Trends, Growth Factors and Opportunities
- 6.3.2. Market Size and Forecast, By Region
- 6.3.3. Market Share Analysis, By Country
- 6.4. Pharmaceutical And Biotechnology Companies
- 6.4.1. Key Market Trends, Growth Factors and Opportunities
- 6.4.2. Market Size and Forecast, By Region
- 6.4.3. Market Share Analysis, By Country
- 6.5. Others
- 6.5.1. Key Market Trends, Growth Factors and Opportunities
- 6.5.2. Market Size and Forecast, By Region
- 6.5.3. Market Share Analysis, By Country
CHAPTER 7: LATERAL FLOW CONJUGATE RELEASE PAD MARKET, BY REGION
- 7.1. Market Overview
- 7.1.1 Market Size and Forecast, By Region
- 7.2. North America
- 7.2.1. Key Market Trends and Opportunities
- 7.2.2. Market Size and Forecast, By Material Type
- 7.2.3. Market Size and Forecast, By Application
- 7.2.4. Market Size and Forecast, By End User
- 7.2.5. Market Size and Forecast, By Country
- 7.2.6. U.S. Lateral Flow Conjugate Release Pad Market
- 7.2.6.1. Market Size and Forecast, By Material Type
- 7.2.6.2. Market Size and Forecast, By Application
- 7.2.6.3. Market Size and Forecast, By End User
- 7.2.7. Canada Lateral Flow Conjugate Release Pad Market
- 7.2.7.1. Market Size and Forecast, By Material Type
- 7.2.7.2. Market Size and Forecast, By Application
- 7.2.7.3. Market Size and Forecast, By End User
- 7.2.8. Mexico Lateral Flow Conjugate Release Pad Market
- 7.2.8.1. Market Size and Forecast, By Material Type
- 7.2.8.2. Market Size and Forecast, By Application
- 7.2.8.3. Market Size and Forecast, By End User
- 7.3. Europe
- 7.3.1. Key Market Trends and Opportunities
- 7.3.2. Market Size and Forecast, By Material Type
- 7.3.3. Market Size and Forecast, By Application
- 7.3.4. Market Size and Forecast, By End User
- 7.3.5. Market Size and Forecast, By Country
- 7.3.6. Germany Lateral Flow Conjugate Release Pad Market
- 7.3.6.1. Market Size and Forecast, By Material Type
- 7.3.6.2. Market Size and Forecast, By Application
- 7.3.6.3. Market Size and Forecast, By End User
- 7.3.7. France Lateral Flow Conjugate Release Pad Market
- 7.3.7.1. Market Size and Forecast, By Material Type
- 7.3.7.2. Market Size and Forecast, By Application
- 7.3.7.3. Market Size and Forecast, By End User
- 7.3.8. UK Lateral Flow Conjugate Release Pad Market
- 7.3.8.1. Market Size and Forecast, By Material Type
- 7.3.8.2. Market Size and Forecast, By Application
- 7.3.8.3. Market Size and Forecast, By End User
- 7.3.9. Italy Lateral Flow Conjugate Release Pad Market
- 7.3.9.1. Market Size and Forecast, By Material Type
- 7.3.9.2. Market Size and Forecast, By Application
- 7.3.9.3. Market Size and Forecast, By End User
- 7.3.10. Spain Lateral Flow Conjugate Release Pad Market
- 7.3.10.1. Market Size and Forecast, By Material Type
- 7.3.10.2. Market Size and Forecast, By Application
- 7.3.10.3. Market Size and Forecast, By End User
- 7.3.11. Rest Of Europe Lateral Flow Conjugate Release Pad Market
- 7.3.11.1. Market Size and Forecast, By Material Type
- 7.3.11.2. Market Size and Forecast, By Application
- 7.3.11.3. Market Size and Forecast, By End User
- 7.4. Asia-Pacific
- 7.4.1. Key Market Trends and Opportunities
- 7.4.2. Market Size and Forecast, By Material Type
- 7.4.3. Market Size and Forecast, By Application
- 7.4.4. Market Size and Forecast, By End User
- 7.4.5. Market Size and Forecast, By Country
- 7.4.6. Japan Lateral Flow Conjugate Release Pad Market
- 7.4.6.1. Market Size and Forecast, By Material Type
- 7.4.6.2. Market Size and Forecast, By Application
- 7.4.6.3. Market Size and Forecast, By End User
- 7.4.7. China Lateral Flow Conjugate Release Pad Market
- 7.4.7.1. Market Size and Forecast, By Material Type
- 7.4.7.2. Market Size and Forecast, By Application
- 7.4.7.3. Market Size and Forecast, By End User
- 7.4.8. India Lateral Flow Conjugate Release Pad Market
- 7.4.8.1. Market Size and Forecast, By Material Type
- 7.4.8.2. Market Size and Forecast, By Application
- 7.4.8.3. Market Size and Forecast, By End User
- 7.4.9. Australia Lateral Flow Conjugate Release Pad Market
- 7.4.9.1. Market Size and Forecast, By Material Type
- 7.4.9.2. Market Size and Forecast, By Application
- 7.4.9.3. Market Size and Forecast, By End User
- 7.4.10. South Korea Lateral Flow Conjugate Release Pad Market
- 7.4.10.1. Market Size and Forecast, By Material Type
- 7.4.10.2. Market Size and Forecast, By Application
- 7.4.10.3. Market Size and Forecast, By End User
- 7.4.11. Rest of Asia-Pacific Lateral Flow Conjugate Release Pad Market
- 7.4.11.1. Market Size and Forecast, By Material Type
- 7.4.11.2. Market Size and Forecast, By Application
- 7.4.11.3. Market Size and Forecast, By End User
- 7.5. LAMEA
- 7.5.1. Key Market Trends and Opportunities
- 7.5.2. Market Size and Forecast, By Material Type
- 7.5.3. Market Size and Forecast, By Application
- 7.5.4. Market Size and Forecast, By End User
- 7.5.5. Market Size and Forecast, By Country
- 7.5.6. Brazil Lateral Flow Conjugate Release Pad Market
- 7.5.6.1. Market Size and Forecast, By Material Type
- 7.5.6.2. Market Size and Forecast, By Application
- 7.5.6.3. Market Size and Forecast, By End User
- 7.5.7. Saudi Arabia Lateral Flow Conjugate Release Pad Market
- 7.5.7.1. Market Size and Forecast, By Material Type
- 7.5.7.2. Market Size and Forecast, By Application
- 7.5.7.3. Market Size and Forecast, By End User
- 7.5.8. South Africa Lateral Flow Conjugate Release Pad Market
- 7.5.8.1. Market Size and Forecast, By Material Type
- 7.5.8.2. Market Size and Forecast, By Application
- 7.5.8.3. Market Size and Forecast, By End User
- 7.5.9. Rest of LAMEA Lateral Flow Conjugate Release Pad Market
- 7.5.9.1. Market Size and Forecast, By Material Type
- 7.5.9.2. Market Size and Forecast, By Application
- 7.5.9.3. Market Size and Forecast, By End User
CHAPTER 8: COMPETITIVE LANDSCAPE
- 8.1. Introduction
- 8.2. Top Winning Strategies
- 8.3. Product Mapping Of Top 10 Player
- 8.4. Competitive Dashboard
- 8.5. Competitive Heatmap
- 8.6. Top Player Positioning, 2023
CHAPTER 9: COMPANY PROFILES
- 9.1. Danaher Corporation
- 9.1.1. Company Overview
- 9.1.2. Key Executives
- 9.1.3. Company Snapshot
- 9.1.4. Operating Business Segments
- 9.1.5. Product Portfolio
- 9.1.6. Business Performance
- 9.1.7. Key Strategic Moves and Developments
- 9.2. AntiTeck
- 9.2.1. Company Overview
- 9.2.2. Key Executives
- 9.2.3. Company Snapshot
- 9.2.4. Operating Business Segments
- 9.2.5. Product Portfolio
- 9.2.6. Business Performance
- 9.2.7. Key Strategic Moves and Developments
- 9.3. Merck KGaA
- 9.3.1. Company Overview
- 9.3.2. Key Executives
- 9.3.3. Company Snapshot
- 9.3.4. Operating Business Segments
- 9.3.5. Product Portfolio
- 9.3.6. Business Performance
- 9.3.7. Key Strategic Moves and Developments
- 9.4. Axiflow Biotech Private Limited
- 9.4.1. Company Overview
- 9.4.2. Key Executives
- 9.4.3. Company Snapshot
- 9.4.4. Operating Business Segments
- 9.4.5. Product Portfolio
- 9.4.6. Business Performance
- 9.4.7. Key Strategic Moves and Developments
- 9.5. Ahlstrom
- 9.5.1. Company Overview
- 9.5.2. Key Executives
- 9.5.3. Company Snapshot
- 9.5.4. Operating Business Segments
- 9.5.5. Product Portfolio
- 9.5.6. Business Performance
- 9.5.7. Key Strategic Moves and Developments
- 9.6. Axiva
- 9.6.1. Company Overview
- 9.6.2. Key Executives
- 9.6.3. Company Snapshot
- 9.6.4. Operating Business Segments
- 9.6.5. Product Portfolio
- 9.6.6. Business Performance
- 9.6.7. Key Strategic Moves and Developments
- 9.7. Porex
- 9.7.1. Company Overview
- 9.7.2. Key Executives
- 9.7.3. Company Snapshot
- 9.7.4. Operating Business Segments
- 9.7.5. Product Portfolio
- 9.7.6. Business Performance
- 9.7.7. Key Strategic Moves and Developments
- 9.8. Cytodiagnostics Inc
- 9.8.1. Company Overview
- 9.8.2. Key Executives
- 9.8.3. Company Snapshot
- 9.8.4. Operating Business Segments
- 9.8.5. Product Portfolio
- 9.8.6. Business Performance
- 9.8.7. Key Strategic Moves and Developments
- 9.9. Advanced Microdevices Pvt. Ltd.
- 9.9.1. Company Overview
- 9.9.2. Key Executives
- 9.9.3. Company Snapshot
- 9.9.4. Operating Business Segments
- 9.9.5. Product Portfolio
- 9.9.6. Business Performance
- 9.9.7. Key Strategic Moves and Developments
- 9.10. Asahi Kasei
- 9.10.1. Company Overview
- 9.10.2. Key Executives
- 9.10.3. Company Snapshot
- 9.10.4. Operating Business Segments
- 9.10.5. Product Portfolio
- 9.10.6. Business Performance
- 9.10.7. Key Strategic Moves and Developments




